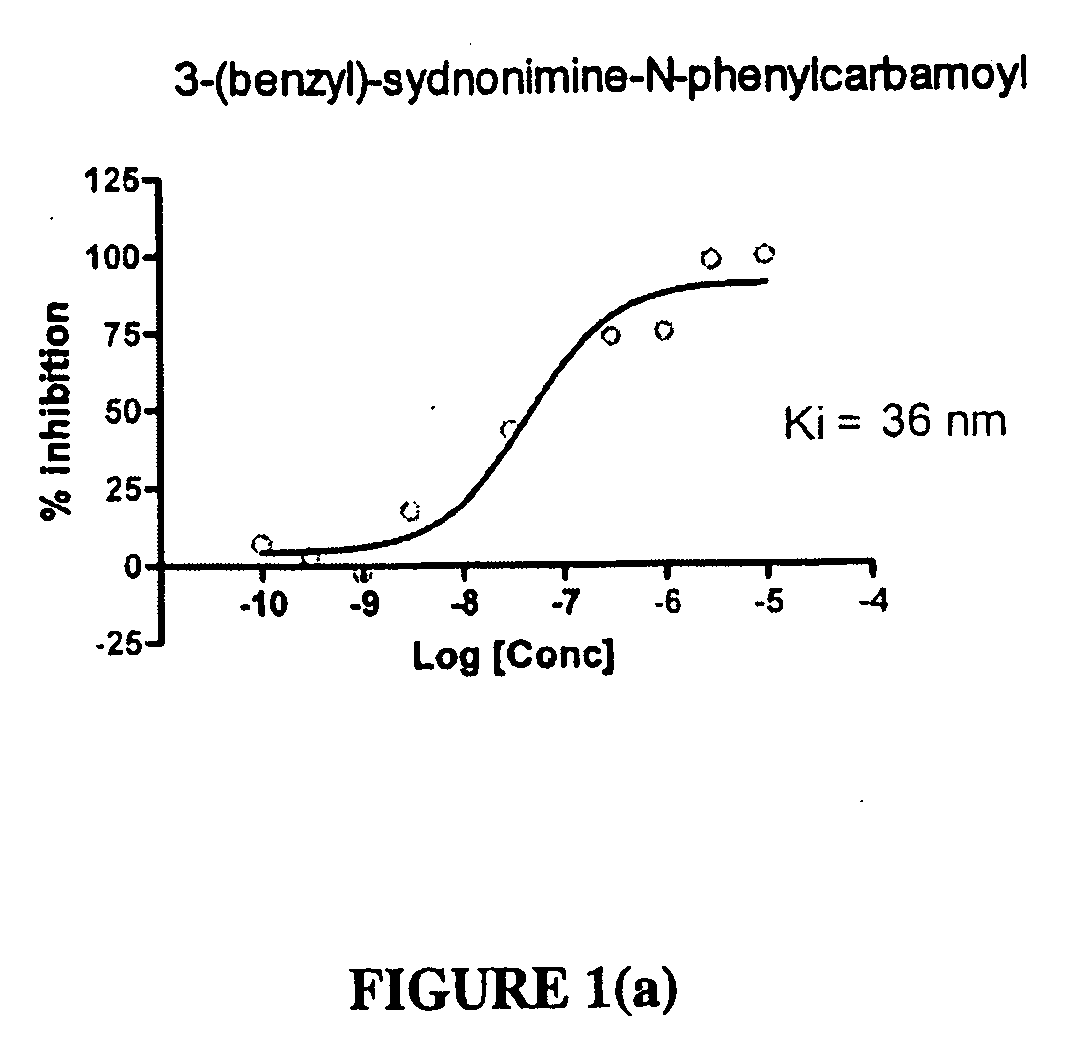
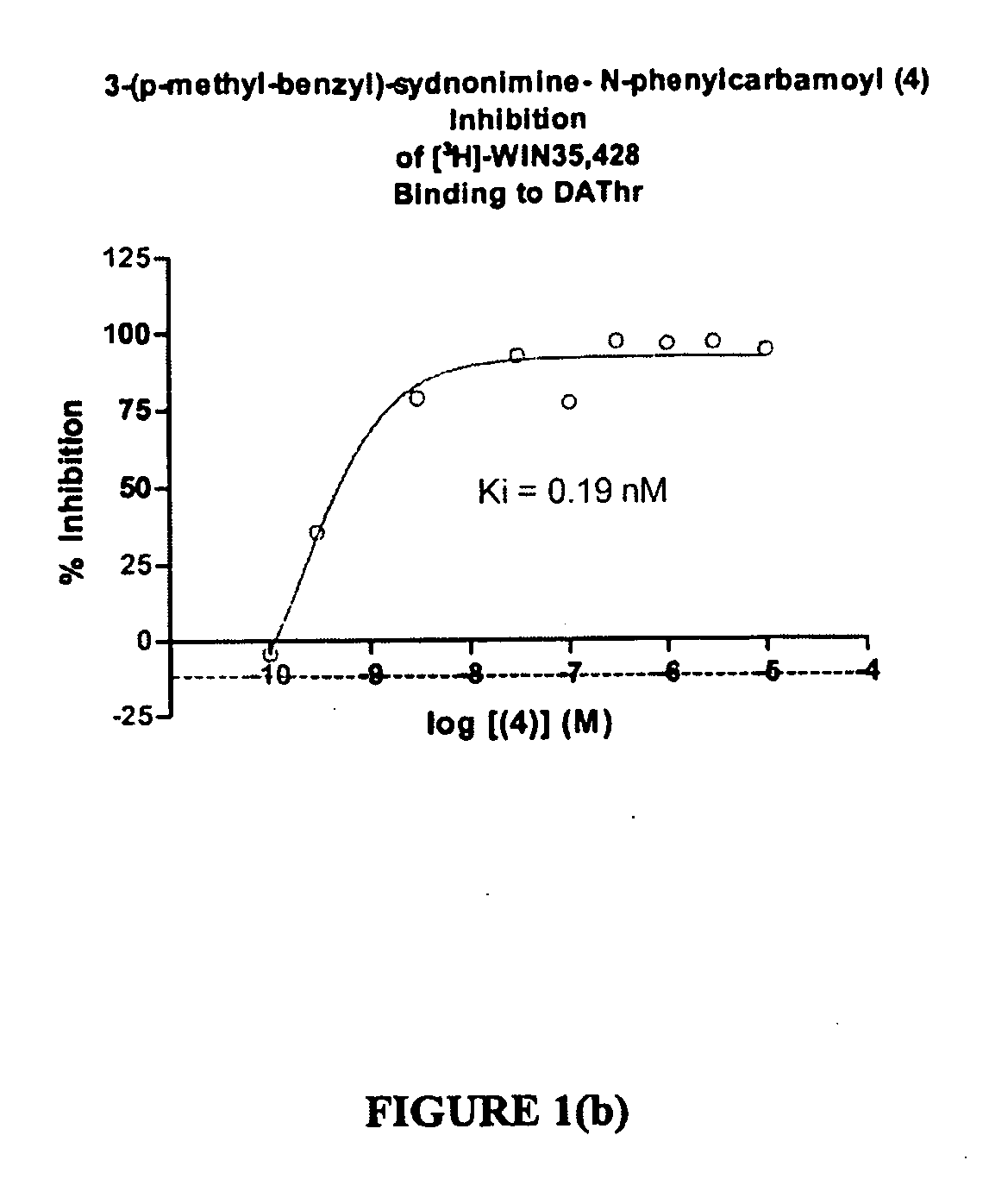
Sydnonimines-specific dopamine reuptake inhibitors and their use in treating dopamine related disorders
a dopamine-related disorder and reuptake inhibitor technology, applied in the field of sydnonimine derivatives, can solve the problems of no long-term efficacy, no long-term efficacy, increased activity or resistance to participate or respond, etc., and achieves no propensity for conversion, suppress locomotor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of N-phenylcarbamoyl-3-(benzyl)-sydonimine
[0092]1.2 ml 7.5N aq. HCl was stirred (at 0° C.) into a mixture of 0.94 g benzyl amine and 0.58 g of potassium cyanide in 2 ml of water. 0.7 g formaldehyde was then added dropwise into the mixture. The resulting mixture was stirred at room temperature for 2 hours and then cooled to 0° C. A solution of 0.62 g sodium nitrite in 1 ml water was added slowly dropwise to the mixture followed by the addition of 1.2 ml 7.5N HCl aq. solution while cooling. The mixture was stirred at room temperature for 1 hour. Ether was used to extract the resulting mixture three times. The combined ether solution was dried with anhydrous sodium sulfate. The solvent was removed under reduced pressure. N-nitroso-benzylaminoacetonitrile (see reaction scheme) was obtained as a yellow oil. The nitroso intermediate was then treated with 500 ml HCl ethereal solution (2.0 M) and stirred for 30 minutes at room temperature. White precipitate was obtained and recr...
examples 2-7
[0093]The compounds N-phenylcarbamoyl-3-(p-carboxylbenzyl-syndonimine (2); N-phenylcarbamoyl-3-(p-methyl-benzyl)-sydnonimine (3); N-(3′,4′-dichlorophenyl) carbamoyl-3-phenethyl sydnonimine (4); N-(3′,4′-dinitrophenyl) carbamoyl-3-p-nitrophenethyl sydnonimine (5); N-phenylcarbamoyl-3-(phenylpropyl)-sydnonimine (6); and N-phenylcarbamoyl-3-p-fluoro-benzyl)-sydnonimine (7) were synthesized following essentially the same procedure as described in Example 1, above, with appropriate substitution of different starting materials in equivalent amounts.
example 8
I. Dopamine Transporter Binding Assay [3H]WIN 35,428
[0094]This assay was carried out according to the procedure described by Javitch, J. J. et al., Mol. Pharmacol. 26: 35-44; 1984
[0095]A. Tissue Preparation (Prepare All Solutions On Ice)[0096]1. Thaw frozen brains from male Guinea Pigs (in assay buffer) and place in 50 mM TRIS-HCl (pH 7.4 at 25° C. with 120 mM NaCl). Isolate striatum.[0097]2. Use a Polytron to homogenize tissue in 20 vols. (w / v) of 50 mM Tris-HCl (pH 7.4 at 25° C. with 120 mM NaCl).[0098]3. Centrifuge homogenate at 48,400×g for approximately 10 minutes at 4° C. Discard supernatant.[0099]4. Wash pellet an additional time as described in steps 2 and 3.[0100]5. Store pellet on ice until needed for binding assay.[0101]6. Using a Polytron (setting 5; approximately 10 seconds) resuspend pellet in 50 mM Tris-HCl (pH 7.4 at 25° C. with 120 mM NaCl) to an initial concentration of 10 mg / ml (original wet weight), such that the final concentration is 8 mg / ml or 4.0 mg tissue / t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com