Hgf-sf monoclonal antibody combinations
a monoclonal antibody and sarcoma cell technology, applied in the field of hgfsf monoclonal antibody combinations, can solve the problems of abnormal tissue regeneration, high levels of human sarcoma cell lines, and abnormal development, and achieve the effect of increasing the binding of antigens and increasing the amount of hepatocyte growth factors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
Anti-HGF / SF Antibody Production
[0093]Murine anti-HGF / SF monoclonal antibodies (Mab) were developed by fusion of the OUR-I myeloma cell line obtained from the American Type Culture Collection (ATCC) with spleen cells of a Balb / C mouse hyper-immunized with native HGF / SF. The fusion was performed when the mouse serum displayed positive neutralizing activity. ELISA positive hybridomas were re-cloned, and neutralizing activity was first screened in the MDCK cell scatter assay. Eight hybridoma cell lines were selected and ascites were produced and purified by FPLC protein-G column (Table 1).
MDCK Scattering Assay
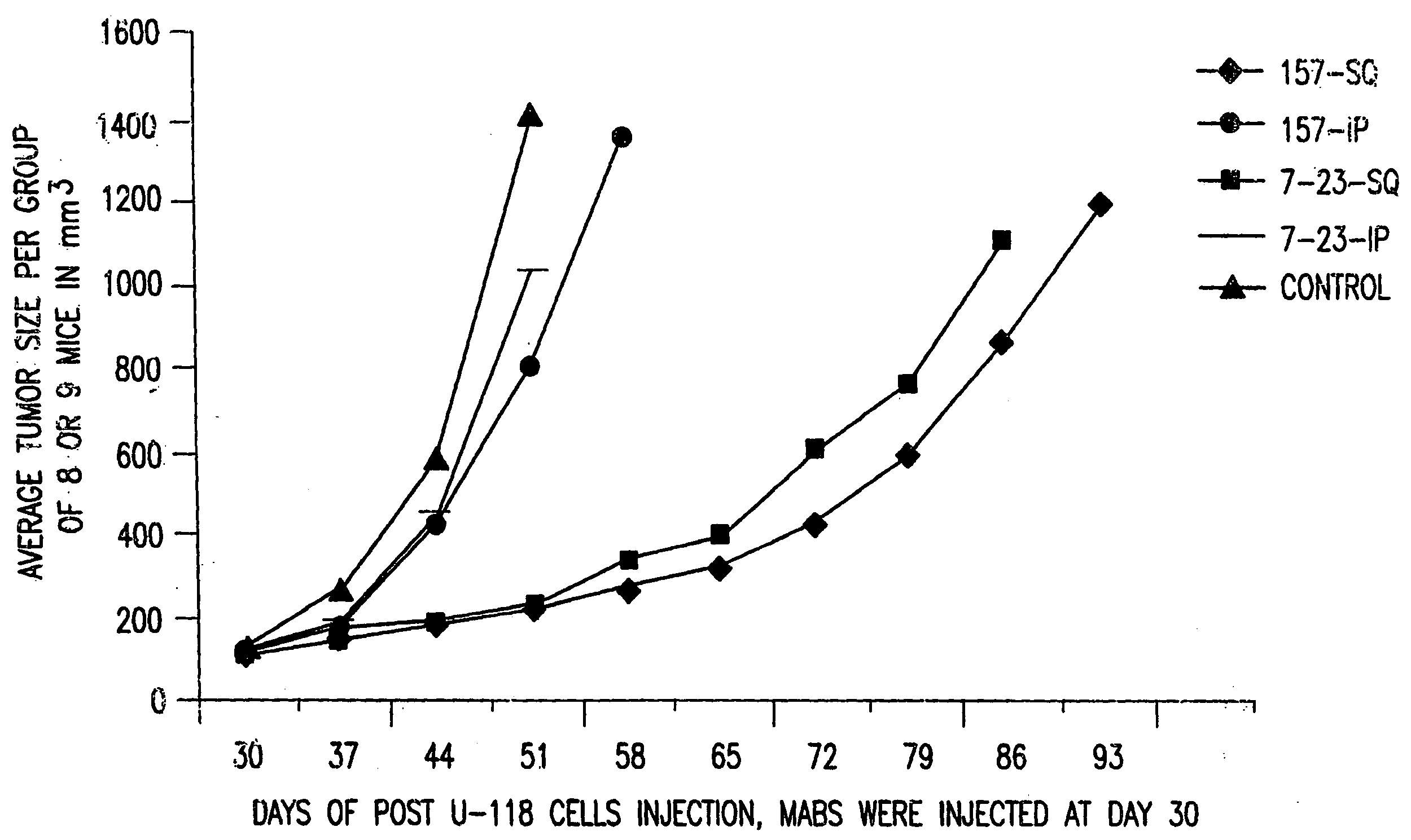
[0094]No single Mab showed significant inhibition of HGF / SF mediated scattering activity in Madin-Darby canine kidney (MDCK) cells while pooled Mabs inhibited MDCK scattering. Whether or not it was possible for a specific sub-set of the Mabs to efficiently neutralize HGF / SF activity was determined. Combinations of two or three of the antibodies were tested and it was determined tha...
example ii
Cell Lines
[0099]MDCK cells were cultured in DMEM medium supplemented with 5% fetal bovine serum (FBS). S-114 cells (transformed with human HGF / SF and Met) (13) were grown in DMEM containing 8% of calf serum. ARZ-2 human renal carcinoma cell line (6) was maintained in DMEM containing 10% FBS. C-127 cell line is NIH 3T3 transformed with human HGF / SF and mouse Met (14), and U-118 cell line is established from human glioma that co-expresses HGF / SF and Met (11). Both cells were maintained in DMEM supplemented with 10% FBS. All cell lines were cultured at 37° C., 5% CO2.
Immunization for Mab Production
[0100]Rabbit polyclonal antibody to HGF / SF was used as positive control. HGF / SF was prepared from S114 cells (15), and mouse Mabs against the ligand were produced by injecting Balb / C mice IP with purified native and denatured (by boiling in sodium dodecyl sulfate (SDS) sample buffer) HGF / SF protein in complete Freund's adjuvant, followed by four additional injections in incomplete Fruend's ad...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| OD | aaaaa | aaaaa |
| v/v | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com