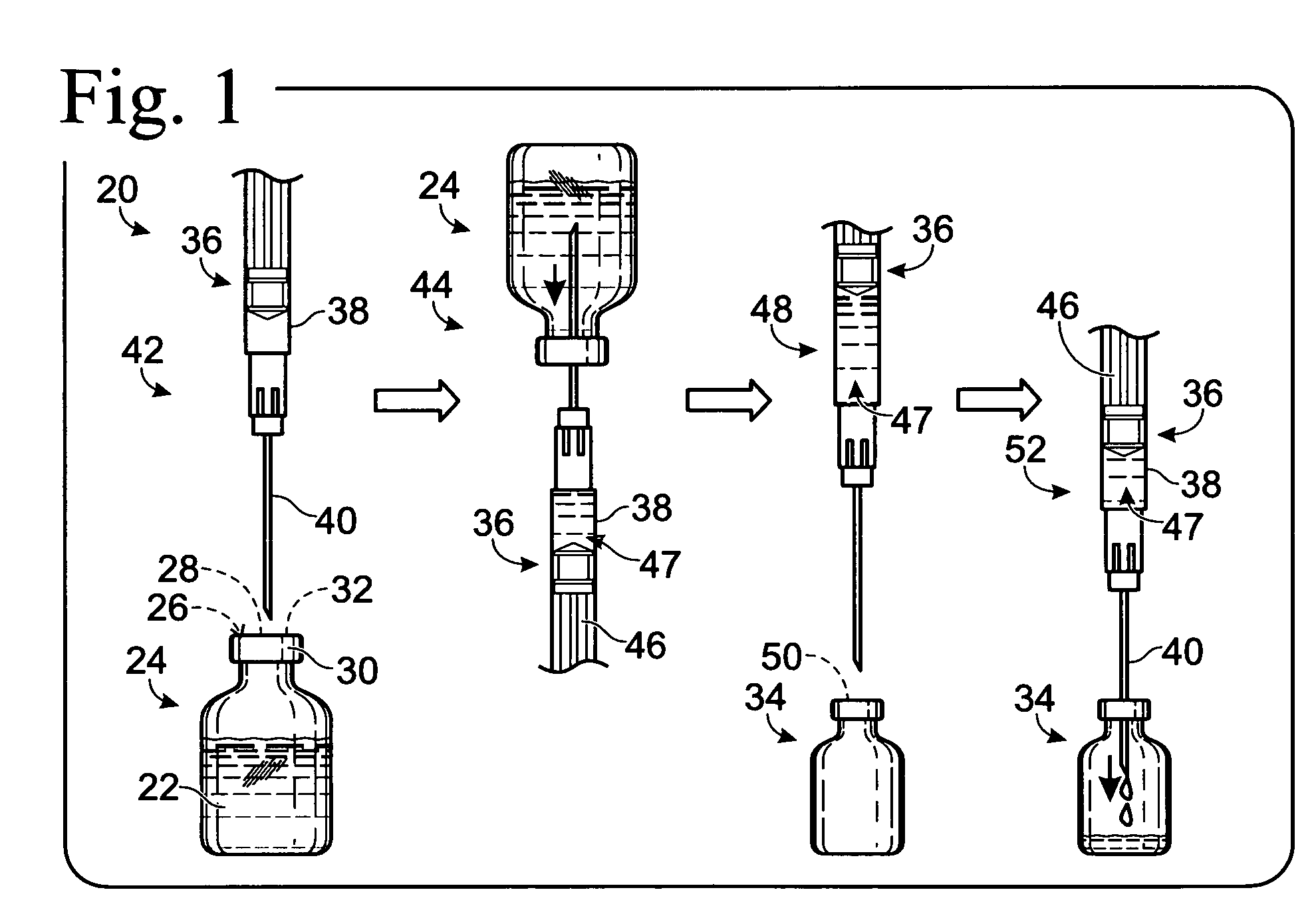
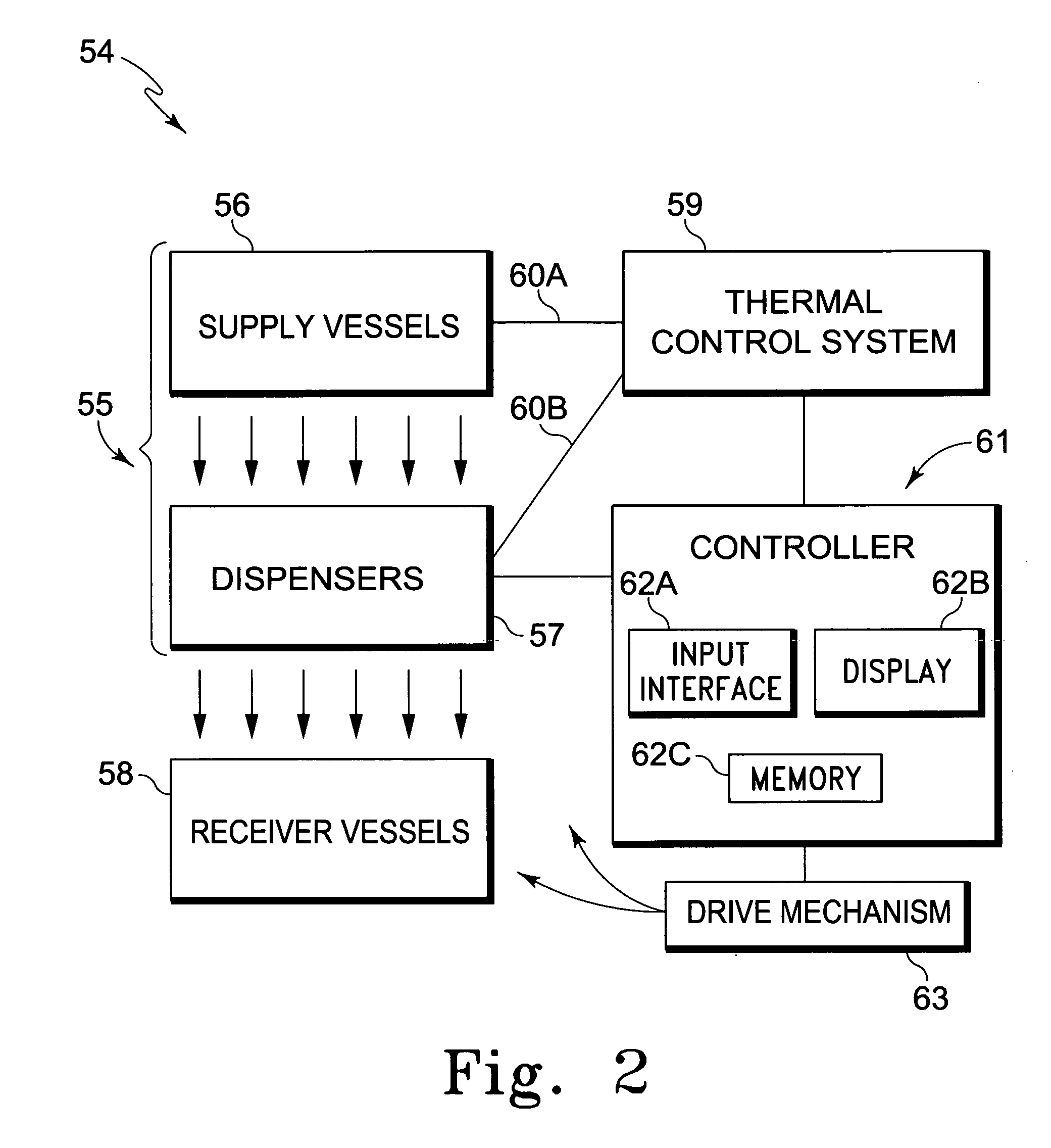
System for dispensing biological fluids
a biological fluid and system technology, applied in the field of biological fluid systems, can solve the problems of physical physical presence of vials, the preparation of allergen mixtures, and unpredictable changes in allergen potency, and affect diagnosis or treatmen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Dispensing System I
[0136]This example describes a first exemplary dispensing system for creating an allergen mixture for immunotherapy by transferring measured volumes of allergen stocks from stock vials to a patient's vial; see FIGS. 8-18.
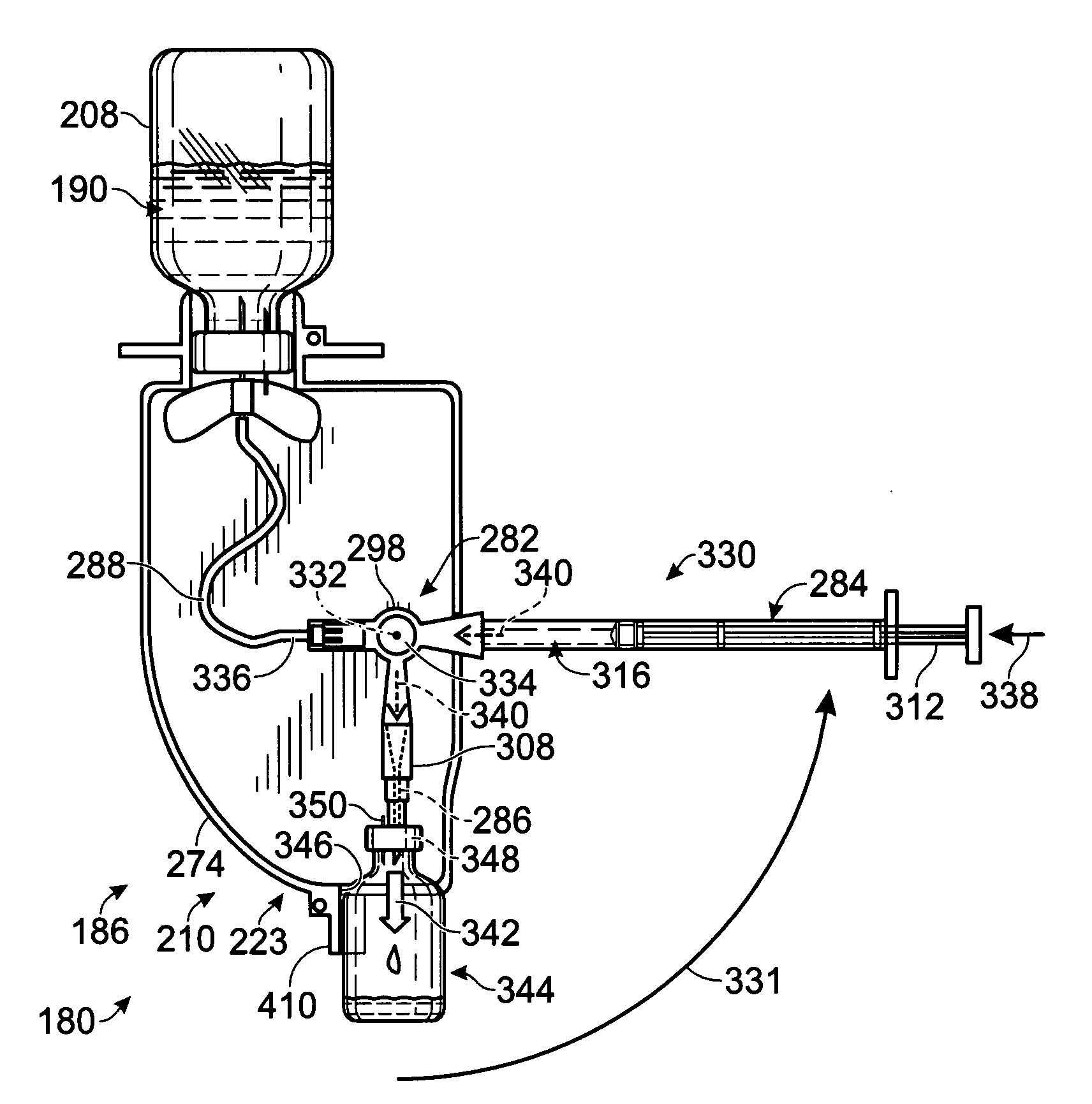
[0137]FIG. 8 show an exemplary system 180 for preparing mixtures of allergens and / or other biological fluids. The system may include a housing 182, a base 184, and a plurality of dispenser stations 186, among others.
[0138]The housing 182 may be coupled pivotably to a base 184 so that the housing can turn around pivot axis 188. Accordingly, the housing may function as a carousel to provide adjustable access to allergen stocks 190 disposed in an interior compartment 192 defined by the walls of the housing. The base may include legs 194 mounted on, and extending upwardly from, a platform 196.
[0139]The housing may include any suitable structure. For example, the housing may include a bottom wall 198, a top wall 200, and a plurality of side walls 202 e...
example 2
Dispensing System II
[0166]This example describes a second exemplary dispensing system 510 for transferring biological fluids between closed vessels; see FIGS. 19-26.
[0167]FIG. 19 shows a dispensing system 510 configured as a floor model. System 510 may be operated manually, to dispense and mix fluids, by an operator(s) standing adjacent the system and / or sitting in a chair adjacent the system, among others. The system may include a housing 512 pivotably coupled to and supported by a pedestal 514 or other support structure.
[0168]The pedestal may include a base platform 516 and a column 518 affixed to the base platform. The column may define an interior compartment 520 in which a power supply 522, electrical connectors (e.g., wires), a cooling mechanism (such as a fan), and / or other electronic and / or system components may be housed. The power supply may be configured to supply electrical power to electrically operated components disposed in housing 512. In some embodiments, the power ...
example 3
Dispenser Station with Climate Control
[0190]This example describes an exemplary dispenser station 710 including a flow system 712 that cools and pressurizes the interior of a dispenser housing 714 of the dispenser station; see FIG. 27.
[0191]Flow system 712 may include a blower mechanism 716, a conduit structure 718, and a filter 720, among others. The blower mechanism may be configured to generate a stream of air 722 that is filtered by filter 720. The filter may be any suitable mechanism for reducing the presence of microorganisms in the air stream, such as a HEPA filter. The conduit structure may provide a flow path for the air stream that extends to dispenser 723 of the dispenser station. For example, the conduit structure may include tubing 724 that carries the air stream from the blower mechanism to the dispenser. The air stream may enter a vessel storage compartment 726 of the dispenser station. Alternatively, or in addition, the air stream may travel through a thermally condu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com