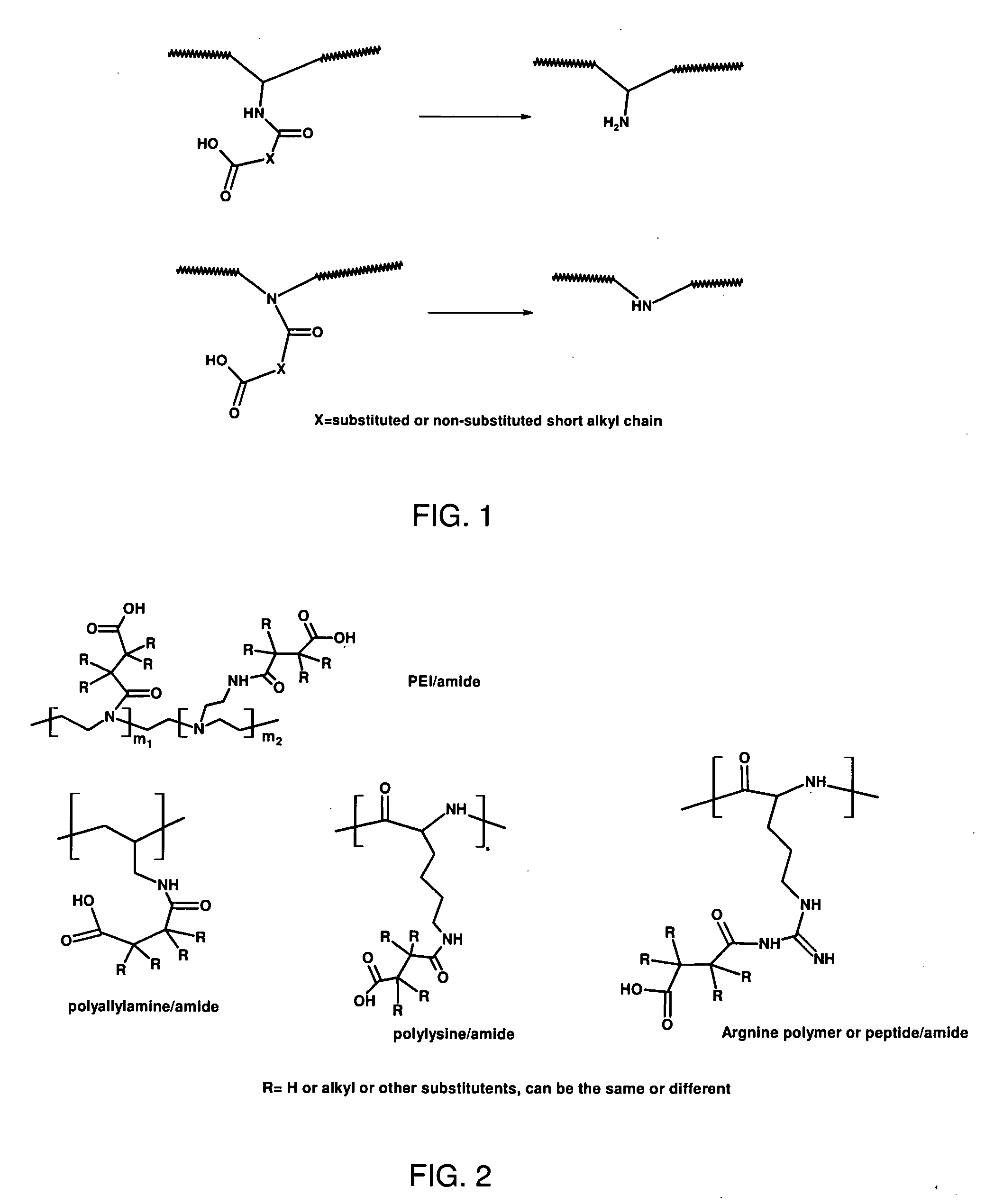
Charge reversible polymers
a charge-reversible polymer and polymer technology, applied in the field of charge-reversible polymers, can solve the problems of severe serum inhibition, inability to reach the targeted tissues other than the liver or intracellular compartment, and limited in vivo applications of positively charged macromolecules or colloidal particles, etc., to achieve rapid hydrolysis, low toxicity, and easy hydrolysis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
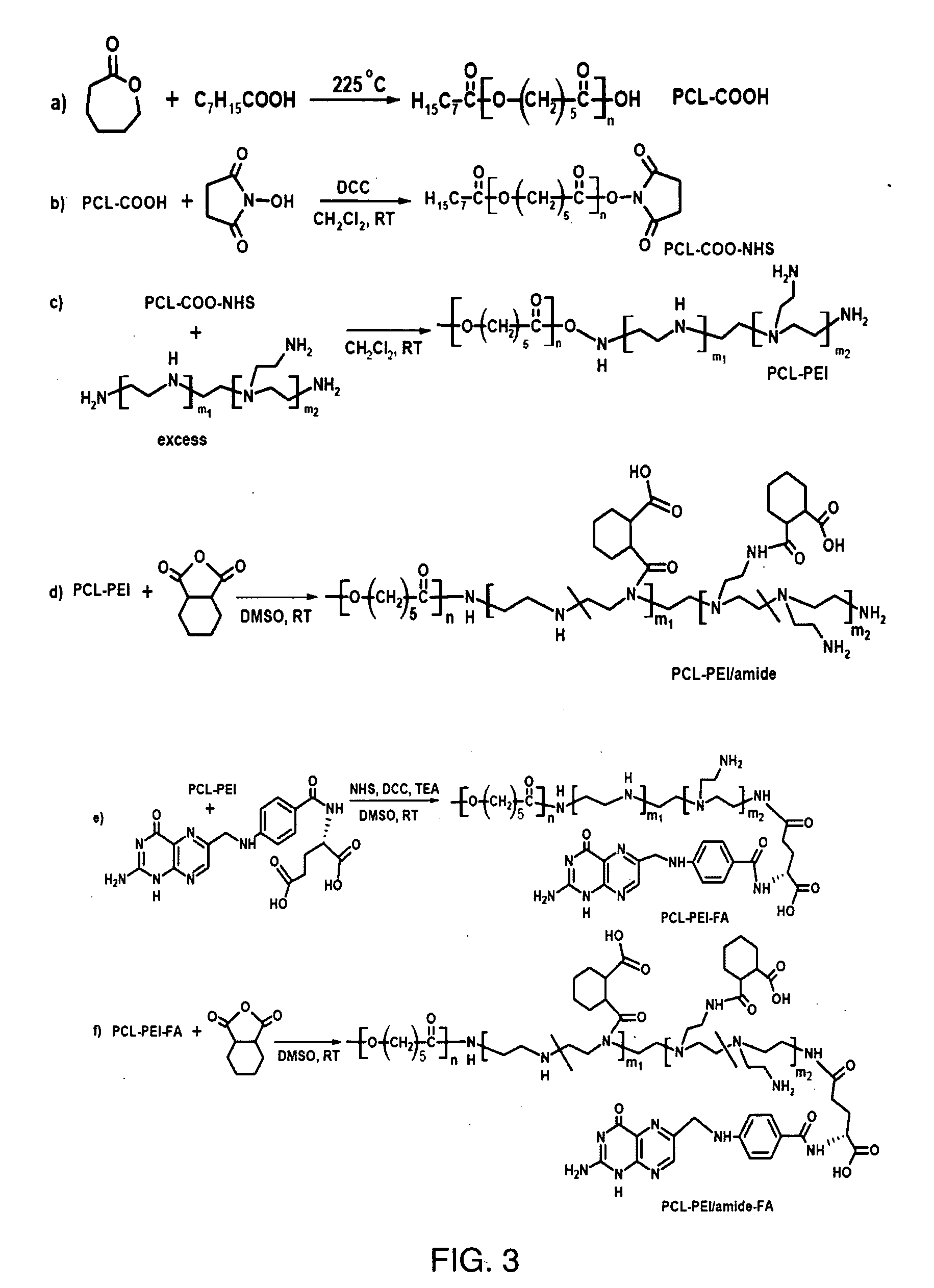
Polymer Synthesis (Scheme 3—FIG. 3)
[0031]Synthesis of poly(ε-caprolactone) (PCL) (a): ε-Caprolactone (ε-CL) (12.5 mL, 113 mmol) and octanoic acid (1.75 mL, 11 mmol) were charged into a flask. The flask was sealed with a rubber septum and degassed. It was heated at 225° C. for 3.5 h with magnetic stirring. The solid was cooled to about 60° C. and dissolved in THF. The solution was poured into 10-fold cold methanol to remove the unreacted monomer. The solid was isolated and purified by reprecipitation. It then was dried under high vacuum at 60° C. PCL with a terminal carboxylic acid (PCL-COOH) (11.8 g, yield 47%) was obtained. 1H-NMR (400 MHz, CDCl3): δ (ppm): 4.08 (t), 2.32 (t), 1.71-1.57 (m), 1.42-1.34 (m), 0.88 (t). Its molecular weight was 3.8 KDa determined by NMR, and 3.2 KDa determined by gel permeation chromatography with polydispersity index of 1.15.
[0032]Synthesis of PCL-COO—NHS (b): PCL-COOH (2.45 g, 0.64 mmol), N-hydroxysuccinimide (NHS, 0.38 g, 3.3 mmol) and 1,3-dicyclohe...
example 2
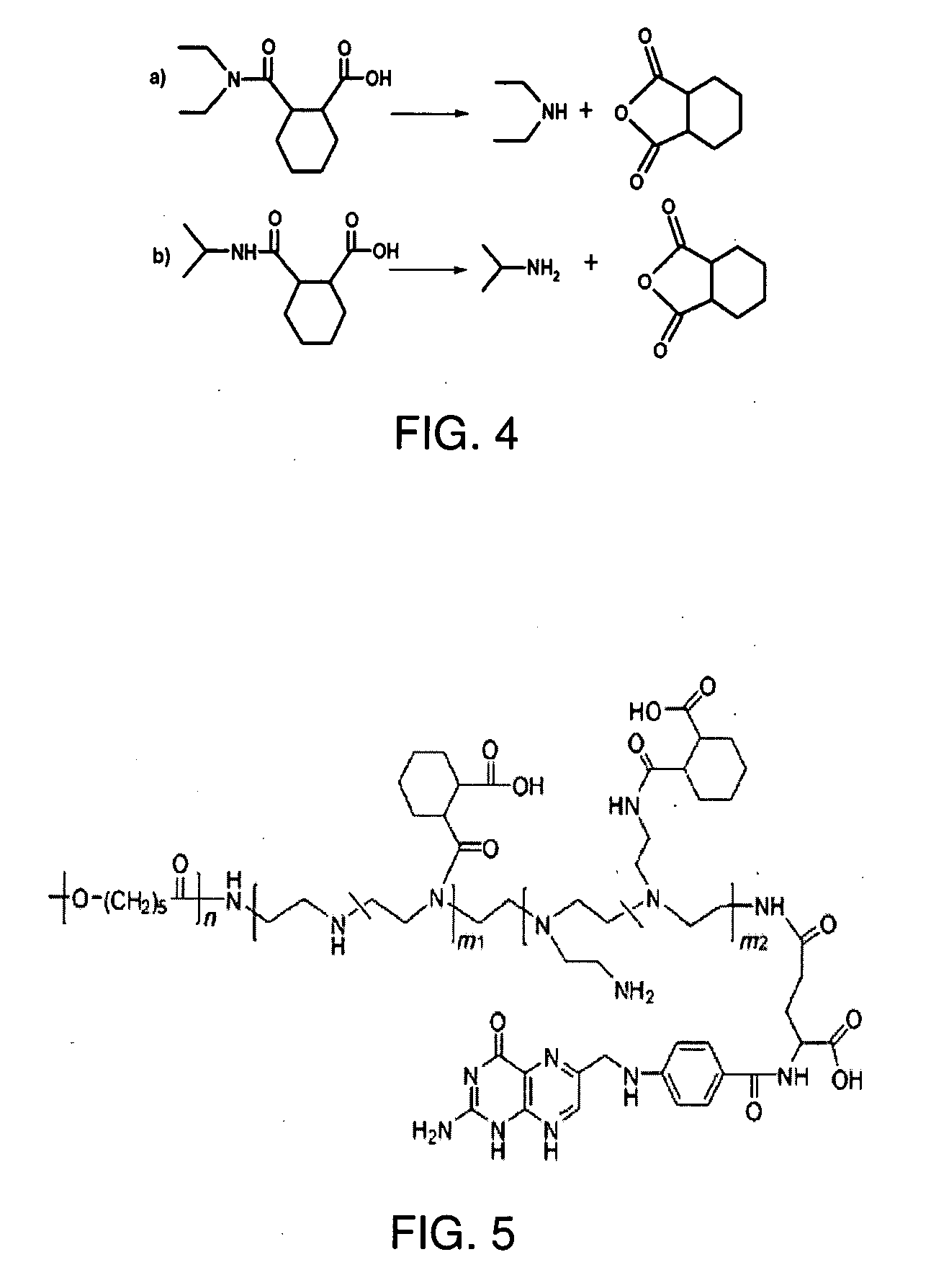
[0037]Model compound synthesis and hydrolytic kinetics measurement (Scheme 4—FIG. 4): Briefly, N,N-diethylamine (415 μL, 4 mmol) and 1,2-cis-cyclohexanedicarboxylic anhydride (617 mg, 4 mmol) were dissolved in 10 mL dichloride methane. The reaction was kept at room temperature for 2 h with stirring. The solvent was then removed by rotary evaporation to obtain the raw product. The raw product was purified by recrystallizing from benzene to get 2-[(diethylamino)carbonyl]cyclohexanecarboxylic acid. 1H NMR (400 MHz, CDCl3): δ (ppm): 3.60-3.22 (m), 2.77 (m), 2.51-2.45 (m), 1.87-1.66 (m), 1.58-1.46 (m), 1.49-1.39 (m), 1.28 (q), 1.17 (t). Similarly, 2-[(isopropylamino)carbonyl]cyclohexanecarboxylic acid was synthesized. 1H NMR (400 MHz, CDCl3): δ (ppm): 3.83 (q), 2.73 (q), 2.64 (q), 1.87 (m), 1.79 (m), 1.64 (m), 1.45 (m), 1.33 (m), 1.01 (d).
[0038]The hydrolysis of the model compounds was monitored by 1H-NMR. Briefly, 2-[(isopropylamino)carbonyl]cyclohexanecarboxylic acid (10 mg) was dissol...
example 3
[0039]The amide hydrolysis kinetics of TCRNs: The hydrolysis of the amides in the TCRNs was monitored by 1H-NMR. The TCRNs in DI water were prepared as described above. The nanoparticle solution was adjusted to pH of 5.0, 6.0 or 7.4, respectively, at a concentration of 1 mg / mL. DMF (1 μl) was added to the solution as the internal standard. These solutions were immersed in a 37° C. water bath. At predesigned time intervals, the TCRN solution (0.5 ml) was sampled and filtered using Centricon centrifugal filter devices (YM-3, 3,000 MWCO, Millipore Corp., Bedford, Mass.). The percentage of hydrolyzed amides was calculated from the integrations of the reference peak at 3.0-2.7 ppm (DMF signal) and the peak at 1.7-1.0 ppm of free 1,2-cis-cyclohexanedicarboxylic acid hydrolyzed from the amides.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com