On-demand hydrogen gas generation device
a hydrogen gas generation and on-demand technology, applied in electrolysis components, electrochemical generators, chemistry apparatus and processes, etc., can solve the problems of hydrogen generation and storage, high pressure hydrogen storage, and occupied space for components
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Positive Electrode Preparation
[0187]This is an example that illustrates the preparation of a suitable positive electrode material, and more specifically illustrates the deactivation of Raney Nickel 3202 and the resulting use thereof to prepare a catalyst layer and ultimately a gas management electrode for use in accordance with the present disclosure:
[0188]A. Deactivation of Raney Nickel
[0189]Raney Nickel is spontaneously combustible in air when it is dry. While it may be possible to process it safely in a wet form, for the purposes of this example, a procedure to deactivate it was used based on literature information (see, e.g., “Novel Methods of stabilization of Raney-Nickel catalyst for fuel cell electrodes”, M. A. Al-Saleh, et. Al., Journal of Power Sources 72 (1998) pp 159-164). The procedure is described in greater detail below.
[0190]Material / Equipment List: Raney 3202 Nickel, slurry in water (Sigma Aldrich product #510068); 5% hydrogen peroxide solution, diluted from 50% solu...
example 2
Correlation Between Hydrogen Gas Generation Rate and Current
[0202]The hydrogen gas generation rate was observed to correlate well with the current measured during generation of gas. Once this correlation was established for different currents, in the interest of simplicity and speed all the reported results were based on current measurements.
[0203]A cylindrical cell design of Example 4 (i.e., the Bobbin-type cell design), further detailed below, was used to make the noted correlation. Individual generator cells were discharged at a fixed current of 350 mA and 650 mA respectively, and the hydrogen generation rates were measured by the displacement of a column of water in a graduated burette. An additional experiment was performed that involved a single cell that was tested at various constant current levels in a stepped manner. A comparison of the theoretical (calculated) vs. the actual measured gas generation rate is shown in the graph presented in FIG. 16, which indicates this corr...
example 3
Spiral-Wound Cell Design
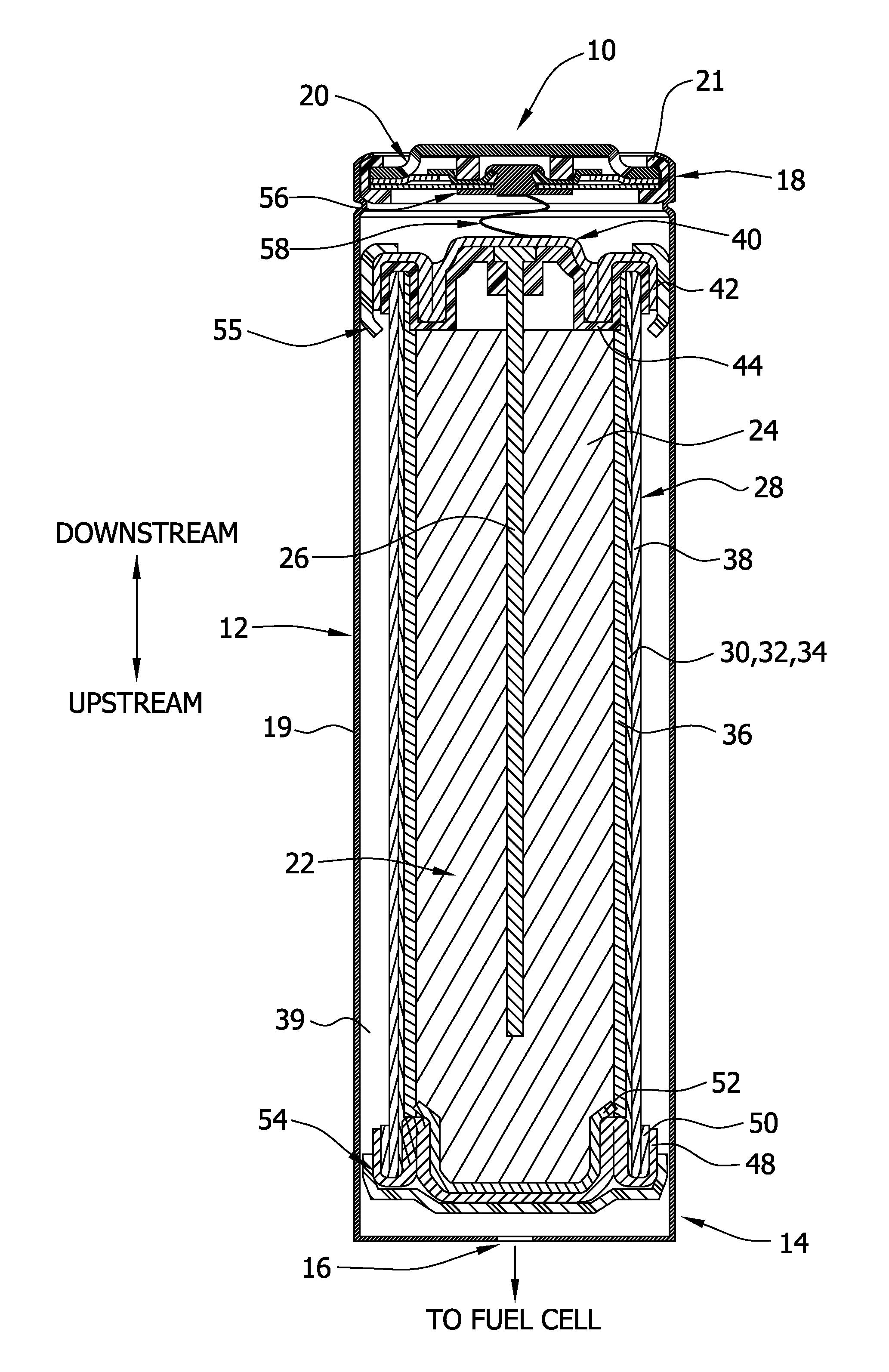
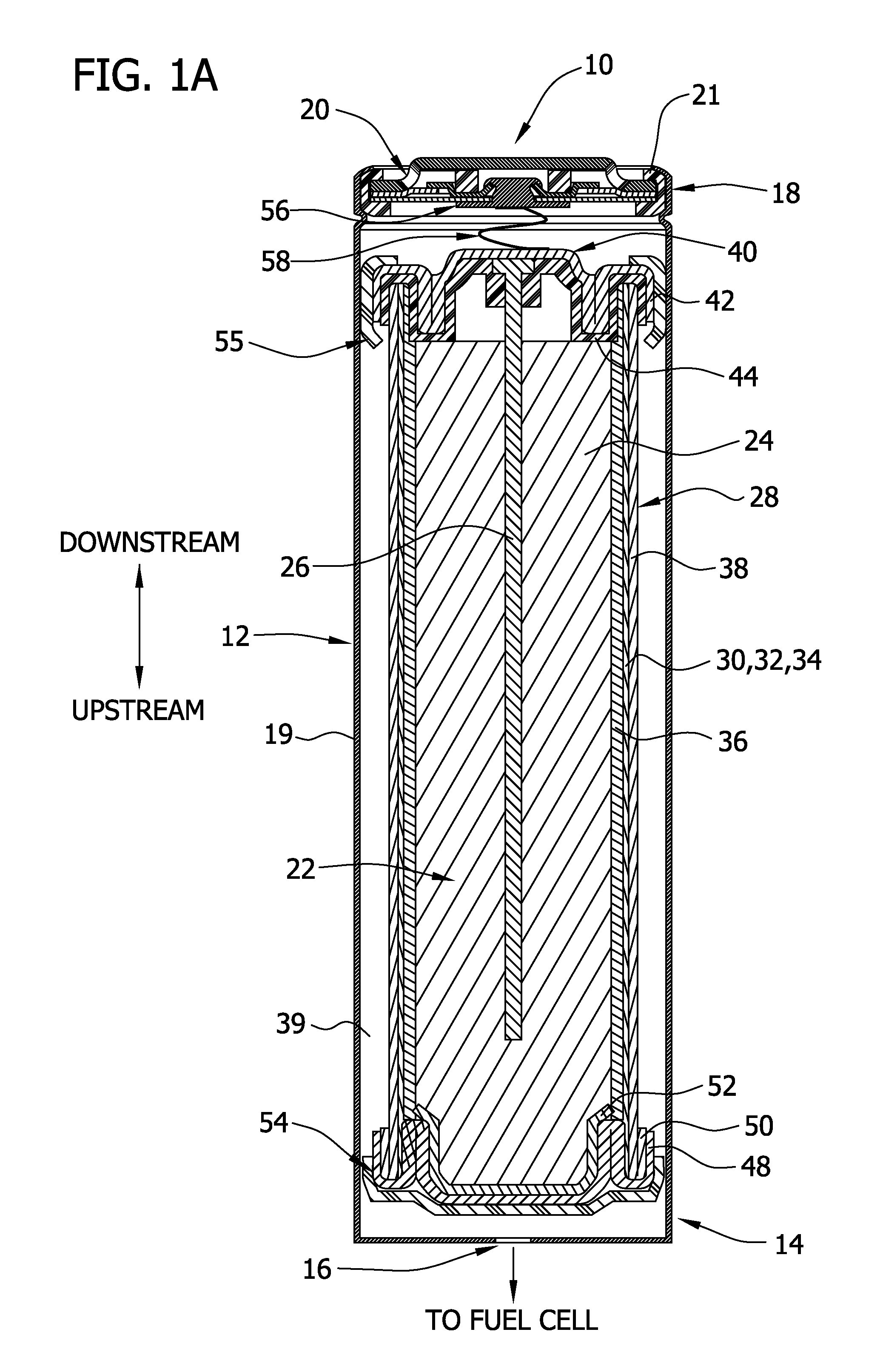
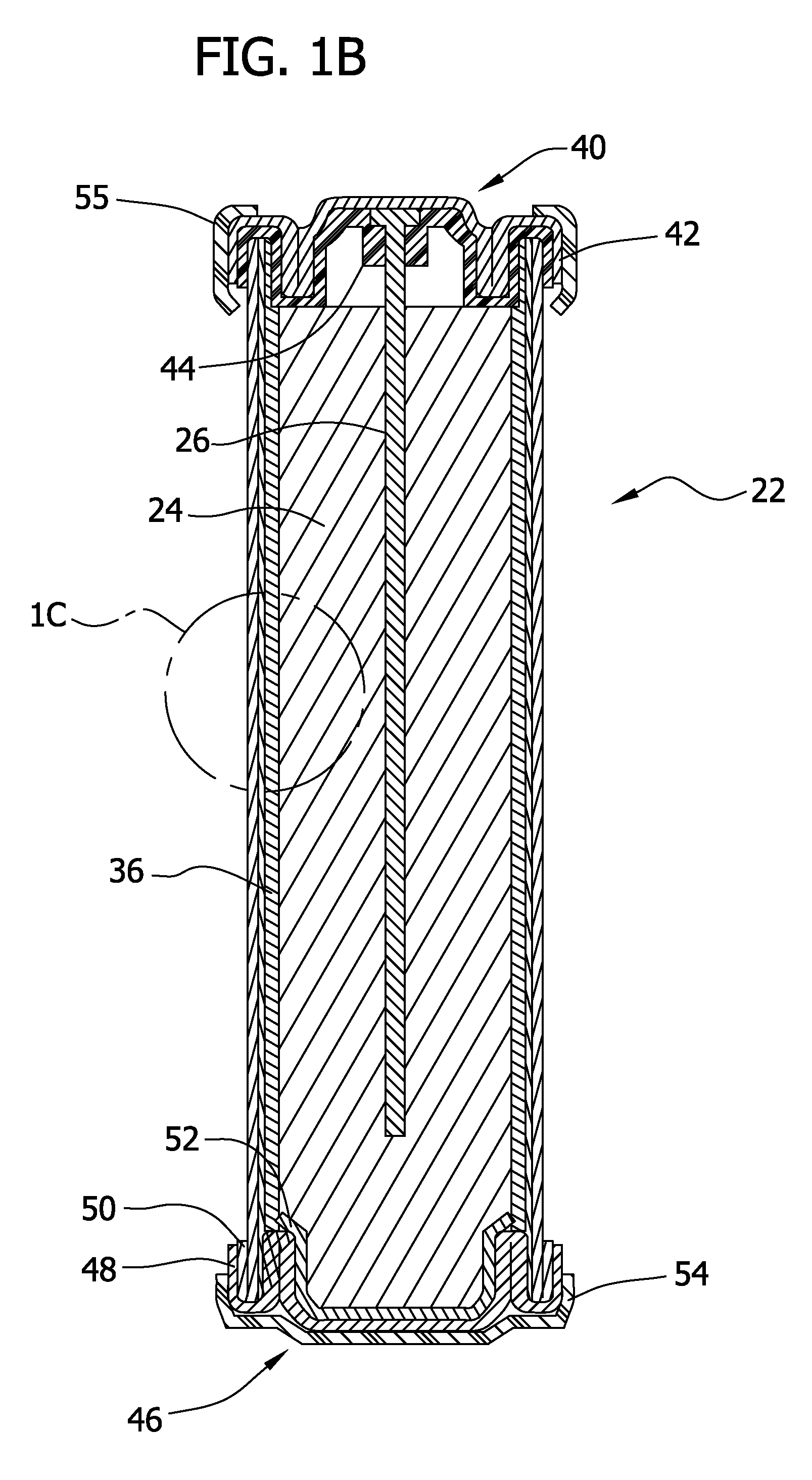
[0204]For this example, a D-size alkaline cell can was used. A spiral wound design is generally known to one skilled in the art to enable higher discharge rates (i.e., discharge currents, and hence gas generation rates in this application) than a typical bobbin cell design. The positive electrode comprised a compressed nickel foam material which was post-plated with nickel to produce a more active nickel surface than the as-received Ni foam (as further detailed herein below). The spiral wound D cell design consisted of a layered arrangement of two positive electrode sheets and three anode pouches made up of the hydrophilic gas impermeable, liquid permeable layer material. The anode pouches comprised 1.5 wraps of 1 mil M2000 and were filled with a gelled Zn anode. The filled anode pouches were about 2 mm thick, to enable a higher anode rate and efficiency than a “bobbin” design used in typical alkaline batteries.
[0205]As noted, the positive electrode for H2 ev...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pressure | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com