Pulmonary delivery of polyene antifungal agents
a technology of antifungal agents and polyene, which is applied in the direction of pharmaceutical delivery mechanism, organic active ingredient delivery, aerosol delivery, etc., can solve the problems of drug degradation, difficult formulation of compounds outside of dry mixing, and technical challenges, and achieve excellent aerosol characteristics, good chemical and physical stability, and efficient administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
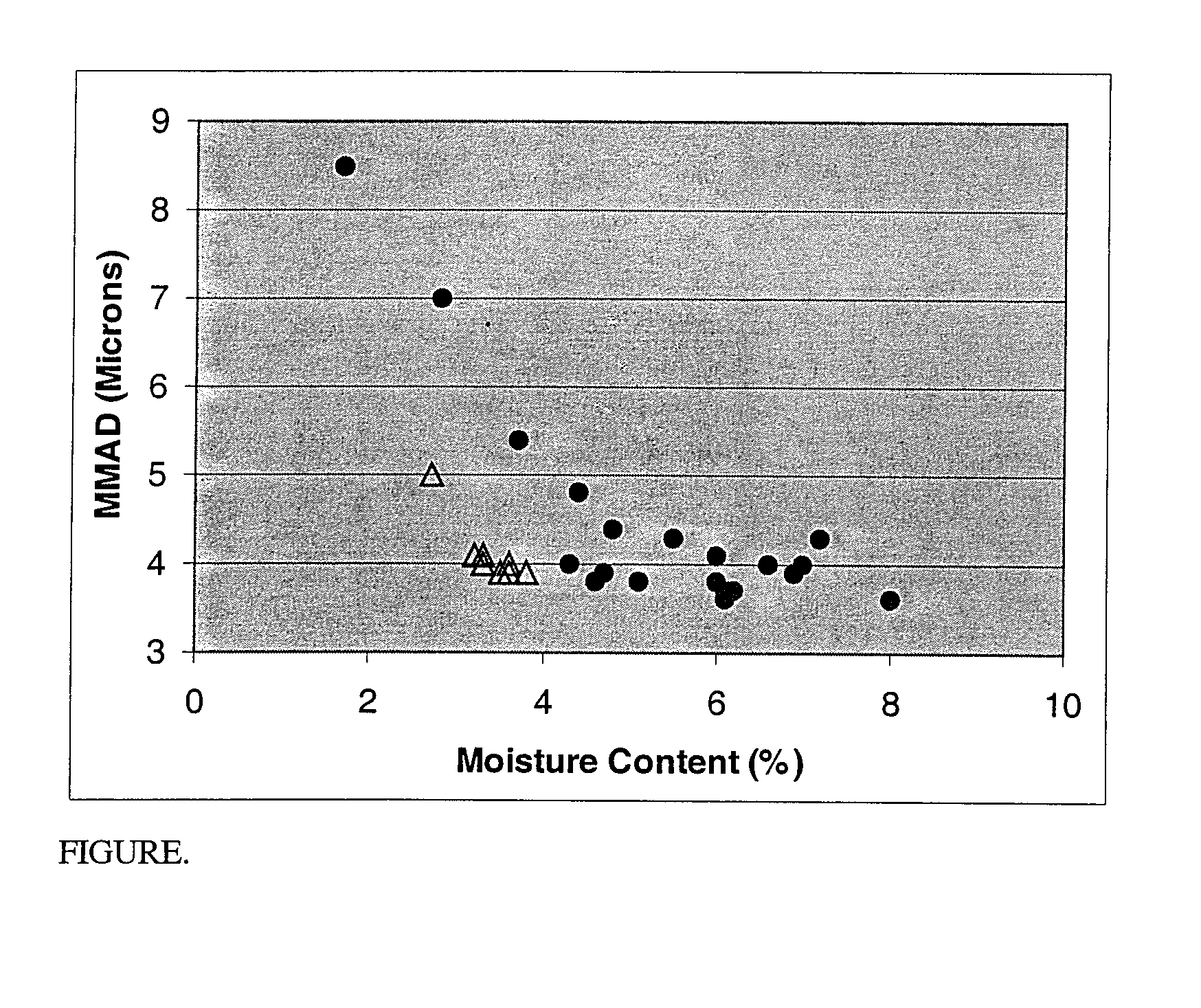
Image
Examples
example 1
Inhaleable Amphotericin B Dry Powder Formulations
[0129]A. Finding a Suitable Solvent for Spray Drying
[0130]The solubility of amphotericin and excipients / additives of interest was determined in various solvents in an attempt to find a solvent system suitable for spray drying (i.e., having a sufficiently high vapor pressure) and capable of dissolving both amphotericin and any added excipients at an extent greater than about 10 mg / mL solvent. Although active agents can be spray-dried as suspensions, having the formulation components dissolved in solution provides resulting particles having a homogeneous composition (i.e., when comparing one particle to another particle)—that is to say, each particle in the composition has approximately the same composition and distribution of formulation components.
[0131]Amphotericin B is difficult to spray dry due to its poor solubility in water at any pH where it is likely to have reasonable stability (amphotericin is insoluble in water at pH 6 to 7)...
example 2
Inhaleable Amphotericin B Dry Powder Formulations Containing Deoxycholate
[0138]A. Preparing Dry Powders
[0139]Sodium deoxycholate was dissolved in water. Amphotericin was added to the sodium deoxycholate solution, and sonicated. 6 molar sodium hydroxide was slowly added to the mixture while stirring and / or sonicating, until the amphotericin was dissolved. The pH of the resulting solution was adjusted (acidified) to 7.0-7.5, while stirring, with 1.2 normal hydrochloric acid. The solution was protected from light. The aim was to utilize the most neutral solution possible that resulted in complete solubilization, to minimize or essentially eliminate any chemical destabilization of the components in the solution. The resulting solution was then spray dried as detailed in Example 1.
[0140]The characteristics of each of the formulations prepared and the characteristics of the resulting powders are provided in Table 3 below.
TABLE 3Amphotericin B / Sodium Deoxycholate Powder Preparation:Formul...
example 3
Inhaleable Dry Powder Formulations of Nystatin
[0142]The solubility of nystatin and leucine in various solvents was explored to identify a solvent for preparing a spray-dried powder of the invention; solubility results are provided in Example 1 above.
[0143]Dry powders were prepared as described in Example 1 above using acidified methanol as the solvent. The characteristics of the resulting powders are summarized below.
TABLE 4Inhaleable Formulations of Nystatin: Composition Characteristics%DrugpH ofResidual% ED ±FormulationLot No.SolutionSolventRSDMMADMorphologyNystatin1696-HS-403.01.674 ± 41.6Dimpledspheres75% Nystatin +1696-HS-423.91.879 ± 31.5Highly dimpled25% L-spheresLeucineMMDDrug Formulation% μmNystatin840.875% Nystatin (w / w)880.625% L-Leucine(w / w)
[0144]Spray drying neat nystatin dissolved in acidified methanol yielded a powder with a good emitted dose of greater than 70% and a superior MMAD of 1.6 microns. The addition of 25% leucine to the formulation resulted in a nominal im...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com