Method for identifying the sequence of one or more variant nucleotides in a nucleic acid molecule
variant technology, applied in the field of identifying the sequence of one or more variant nucleotides in a nucleic acid molecule, can solve the problems of high specificity, high cost and time-consuming procedure of sequencing the whole genome of individuals,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Methods
[0049]Materials. SURVEYOR nuclease (CEL II) was purified from celery by a modification of known methods (Yang, et al. (2000) supra; Gerard, et al. (2006) supra). Enzymatic activity was assigned based upon a denatured DNA solubilization assay performed at pH 8.5 (Yang, et al. (2000) supra). One unit of solubilization activity was defined as the amount of enzyme required to produce 1 ng of acid-soluble material in 1 minute at 37 ° C.
[0050]OPTIMASE® Polymerase and MAXIMASE™ Polymerase were from Transgenomic, Inc (Omaha, Nebr.). M-280 Streptavidin magnetic DYNABEADS® were from Dynal Biotech (INVITROGEN, Carlsbad, Calif.). Cloned T4 DNA ligase was prepared by Transgenomic, Inc. Streptavidin was purchased from SIGMA (Sigma / Aldrich, St. Louis, Mo.). Control G and Control C plasmids were from Transgenomic, Inc. Control C and Control G have inserts (632 bp) that differ at a single base pair, so that annealing of their PCR products produces heteroduplices that when cleave...
example 2
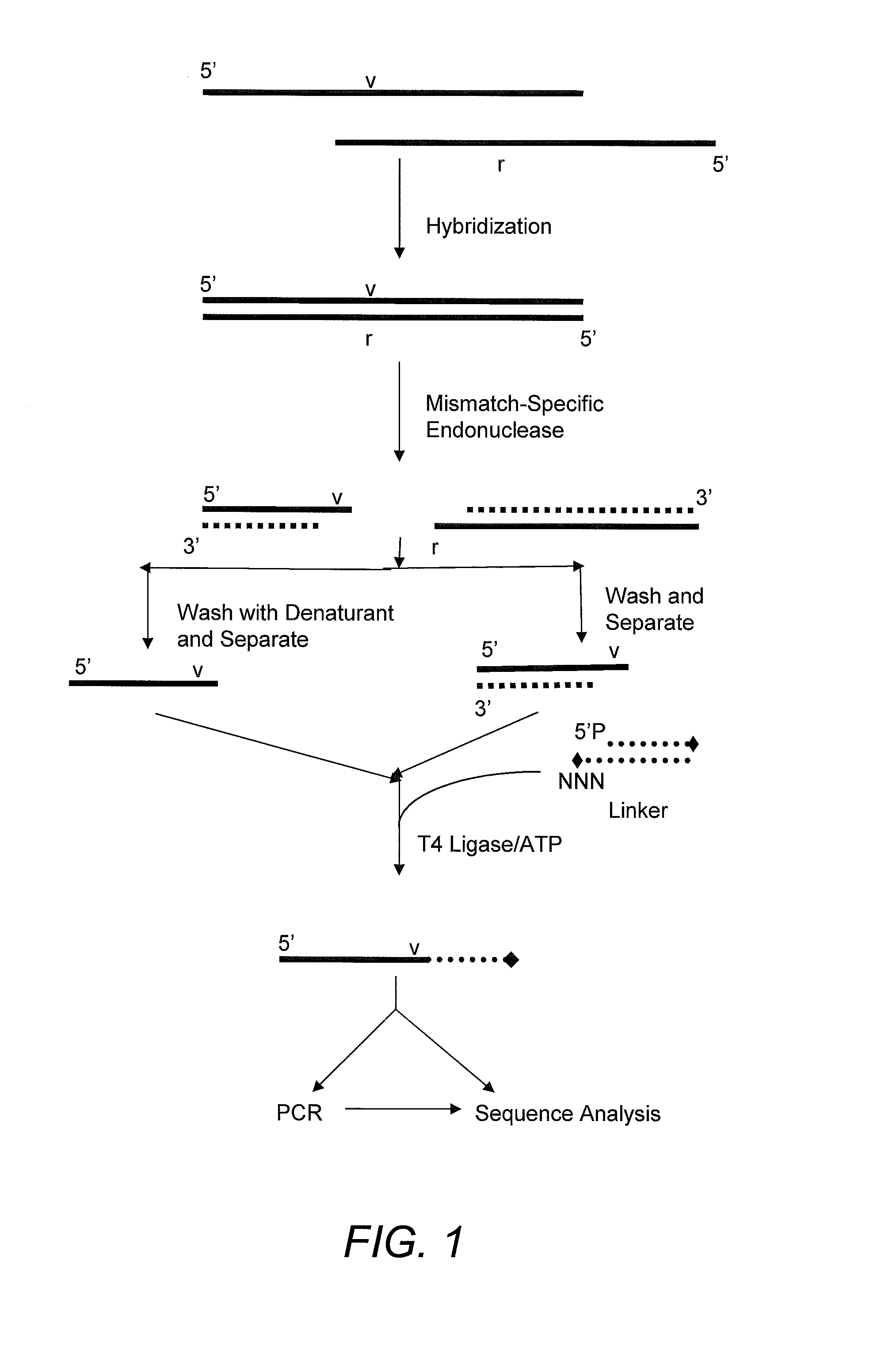
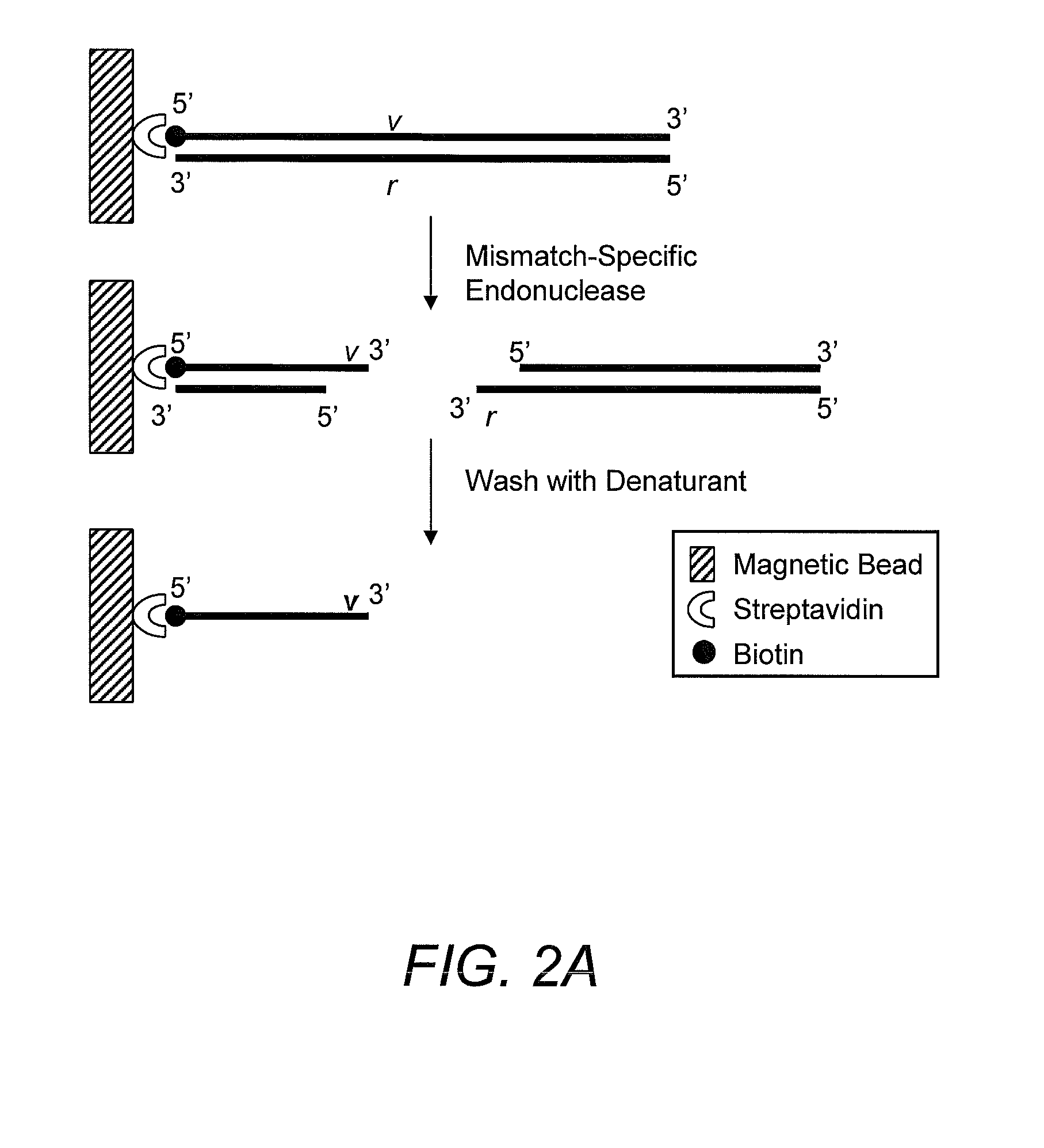
Capturing 3′-Overhang Nucleotides Generated by Mismatch Endonuclease Using Randomized Terminal Linker-Dependent PCR
[0062]Requirements for Capturing the 3′-Overhang Nucleotides Generated by SURVEYOR Nuclease at Mismatch Cut Sites. Direct sequence analysis of SURVEYOR nuclease cleavage products involves the capture of the 3′-overhangs created after digestion at a mismatch site. The capture must be specific to eliminate background during the subsequent sequencing reaction. The main challenge comes from background produced as the result of the 5′-to-3′ exonuclease activity of SURVEYOR nuclease (Gerard, et al. (2006) supra) attacking the ends of PCR products. After digestion of a Control G / C heteroduplex with SURVEYOR nuclease, cleavage fragments (415 bp and 217 bp) are present in a mixture along with undigested homoduplices and heteroduplices (632 bp) and the 5′-to-3′ exonuclease activity of SURVEYOR nuclease creates 3′-overhangs at both ends of the full-length molecules that will compe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com