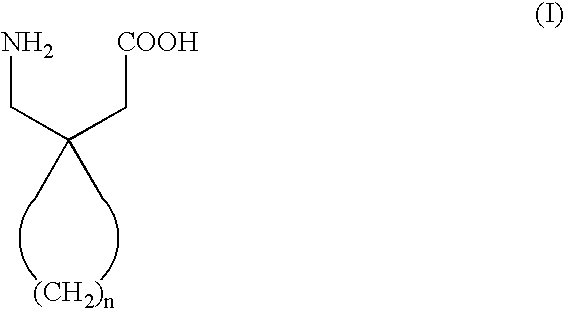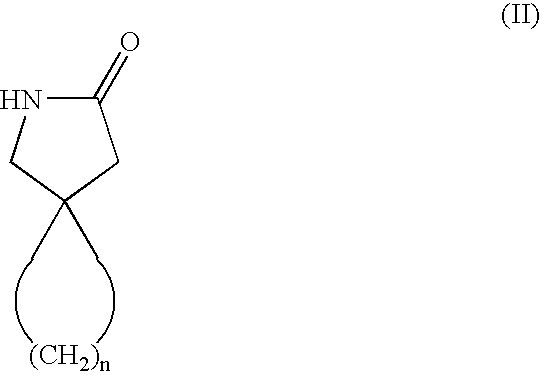Process for obtaining cyclic amino acids
a technology amino acids, which is applied in the field of industrial processes for obtaining cyclic amino acids, can solve the problems of long time used to reduce the content of mineral acid anion to sufficiently low values, the stability of cyclic amino acids is not good, and the disadvantage of industrial scal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
Example 1 Preparation of Gabapentin
[0100]An aqueous solution comprising 200 kg of gabapentin hydrochloride and 1,500 kg of deionized water is loaded in the cathodic compartment, and 5,000 kg of an aqueous solution of sulfur dioxide at 0.5% by weight is loaded in the anodic compartment in an electrochemical reactor with a useful area of 6 m2 equipped with an anodic compartment having oxygen-evolving anodes and with a cathodic compartment with stainless steel cathodes, separated by a selective anionic membrane SYBRON MA series (marketed by Sybron Chemicals).
[0101]The circulation pumps are started up and a constant voltage of 11 V is set for the electrolysis.
[0102]The values of the following parameters are recorded during the electrochemical process: density of the current intensity, conductivity and pH of the cathodic compartment.
[0103]The density of the intensity gradually decreases from the initial value of 1,250 A / m2 to a final value of 50 A / m2. The conductivity of the cathodic com...
PUM
| Property | Measurement | Unit |
|---|---|---|
| voltage | aaaaa | aaaaa |
| voltage | aaaaa | aaaaa |
| voltage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com