Medical device for repair of tissue and method for implantation and fixation
a technology of medical devices and tissue, applied in the field of medical devices for tissue repair, can solve the problems of cartilage wear on the condylar or tibial plateau surface, fibrocartilage deterioration, and possible tear, and achieve the effect of securing the filamen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
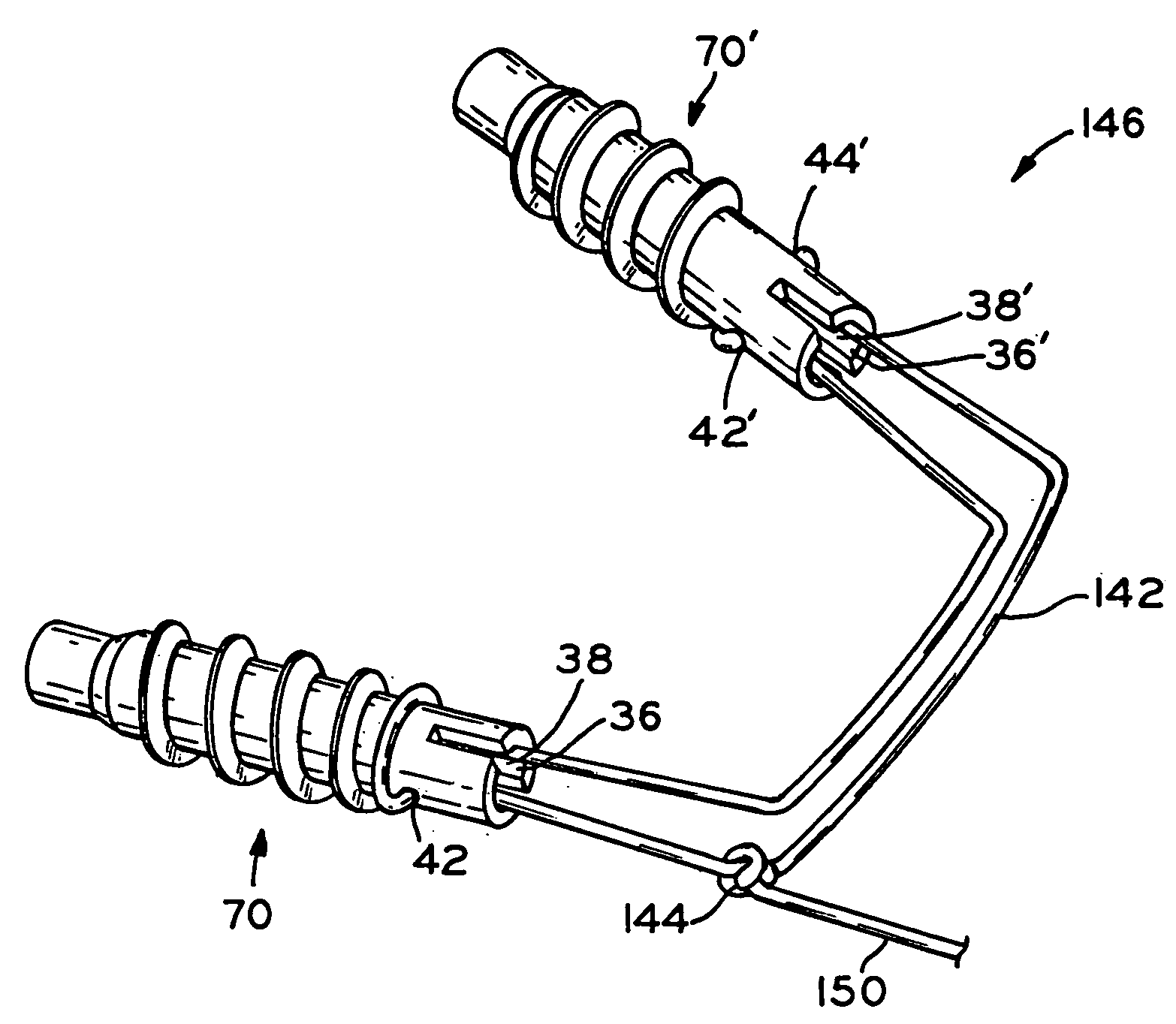
[0045]FIG. 7 shows conduit 30 according to one embodiment of the present invention. Conduit, as used herein, means only an elongate body and does not define any other structural features. Conduit 30 includes a first end 32, a second end 34, and through bore 36 extending from first end 32 to second end 34. In one embodiment, bore 36 has a non-circular cross-section. Body 30 can be manufactured from any biocompatible material. Body 30 has a length from first end 32 to second end 34 as small as 2 mm, 3 mm, or 4 mm and as large as 10 mm, 12 mm, or 15 mm. Additionally, conduit 30 may be coated with biocompatible substances to facilitate tissue regeneration, improve circulation, or achieve any other biologically desirable responses. For example, interior 38 of through bore 36 may be coated with an anti-coagulant to prevent coagulation of blood within through bore 36, thereby promoting the delivery of blood to a torn or damaged tissue area. Alternatively, bore 36 may contain a scaffold mat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com