Compounds for Nonsense Suppression, Use of These Compounds for the Manufacture of a Medicament for Treating Somatic Mutation-Related Diseases
a technology for somatic mutations and compounds, applied in the field of compound for suppressing nonsense, to achieve the effect of inhibiting the growth of cancer cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Compounds of the Invention
A. Scheme A:
[0333]3-(6-methyl-benzooxazol-2-yl)-benzoic acid (Compound 3) and similar compounds of the invention may be generally prepared according to Scheme A as follows.
Synthesis of 3-Cyano-benzoic acid methyl ester (I)
[0334]A solution of 3-cyanobenzoic acid (3.15 g, 21.41 mmol) and K2CO3 (4.85 g, 35.48 mmol) in DMF (30 ml) is treated with iodomethane (4.56 g, 32.11 mmol) and stirred at room temperature for 5 h until the complete consumption of the starting material. The reaction mixture is poured into 120 ml of ice-water to precipitate solid. The solid is recrystallized from 100 ml of water-methanol to provide 2.6 g (75.3% yield) of the product as a white crystalline solid; 1H NMR (300 MHz, CDCl3): δ 8.32 (t, 1H), δ 8.26 (td, J1=7.9, J2=1.7, 1H), δ 7.72 (td, J1=7.6, J2=1.7, 1H), δ 7.61 (dt, J1=7.7, J2=7.6, 1H), δ 3.96 (s, 3H); MS+=162.
Synthesis of 3-methoxycarboniumidoyl-benzoic acid methyl ester hydrochloride (II)
[0335]3-Cyano-benzoic ac...
example 2
Nonsense Suppression Activity
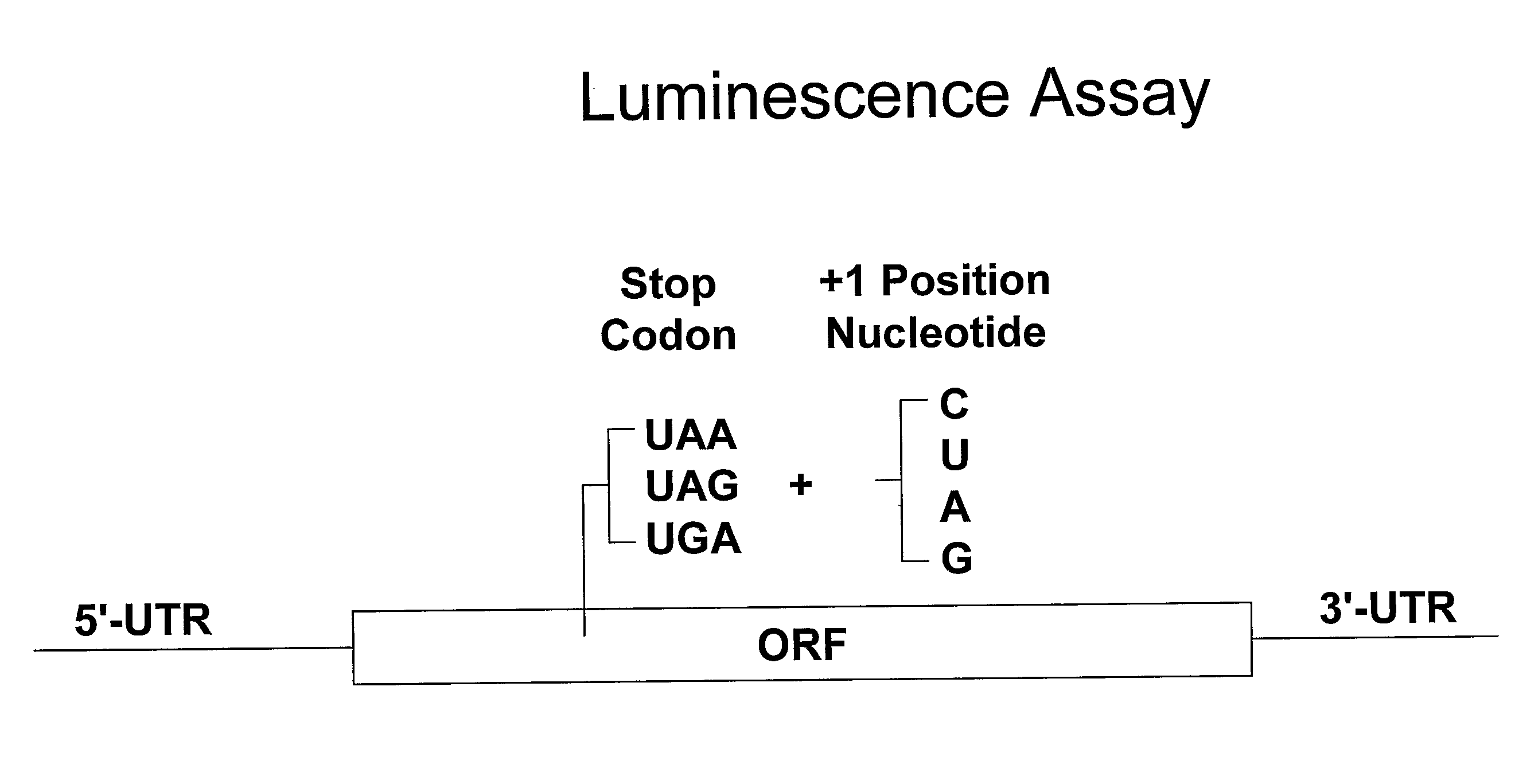
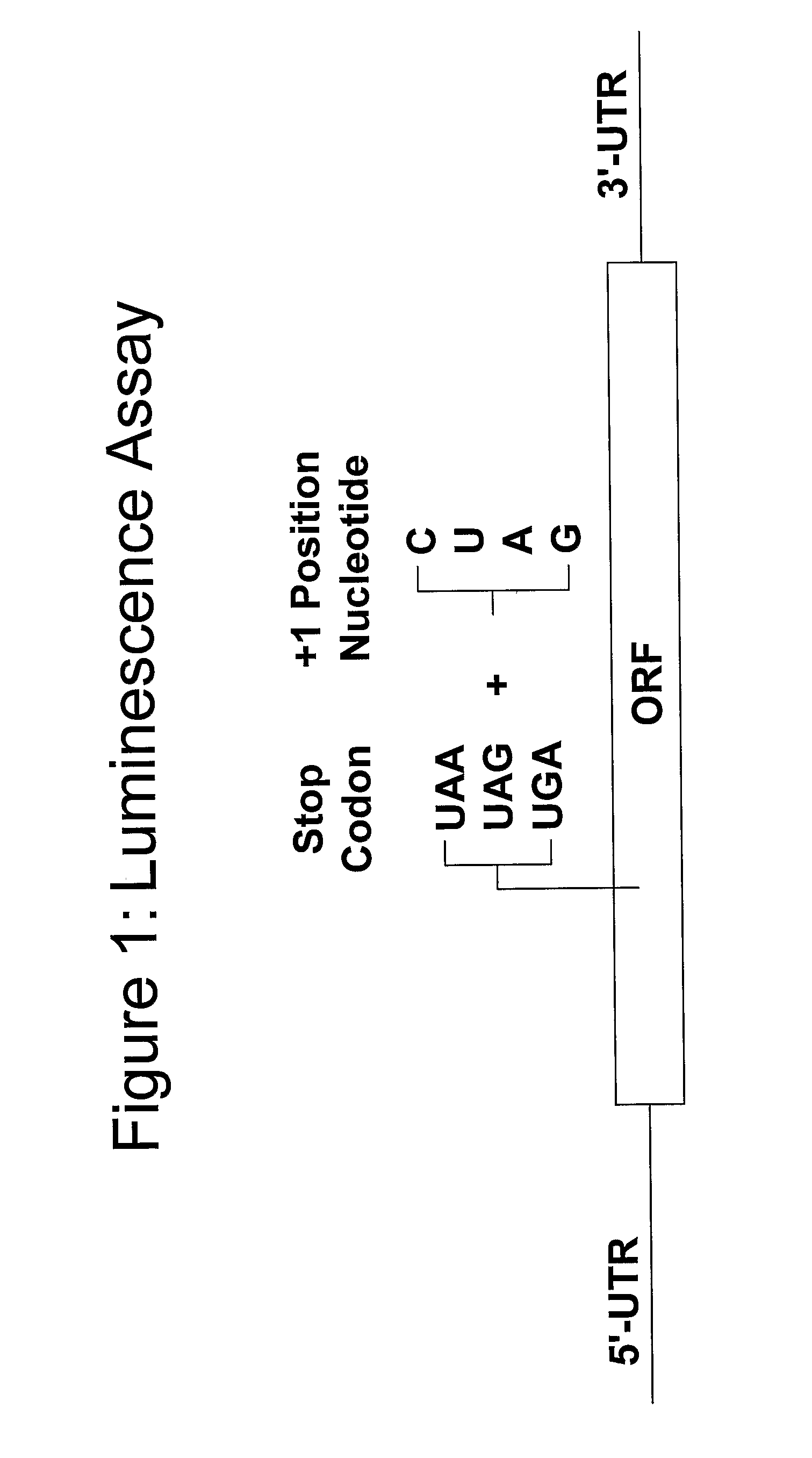
[0371]A functional, cell-based translation assay based on luciferase-mediated chemoluminescence (International Application PCT / US2003 / 023185, filed on Jul. 23, 2003, hereby incorporated by reference in its entirety) permits quantitative assessment of the level of nonsense suppression. Human embryonic kidney cells (293 cells) are grown in medium containing fetal bovine serum (FBS). These cells can be stably transfected with the luciferase gene containing a premature termination codon at amino acid position 190. In place of the threonine codon (ACA) normally present in the luciferase gene at this site, each of the 3 possible nonsense codons (TAA, TAG, or TGA) and each of the 4 possible nucleotides (adenine, thymine, cytosine, or guanine) at the contextually important downstream +1 position following the nonsense codon are introduced by site-directed mutagenesis. As such, amino acid 190 in the luciferase gene containing a premature termination codon is TAA,...
example 3
Readthrough Assay
[0374]A functional, cell-based translation assay based on luciferase-mediated chemoluminescence (International Application PCT / US2003 / 023185, filed on Jul. 23, 2003 and incorporated by reference in its entirety) permits assessment of translation-readthough of the normal stop codon in a mRNA. Human embryonic kidney cells (293 cells) are grown in medium containing fetal bovine serum (FBS). These cells are stably transfected with the luciferase gene containing a premature termination codon at amino acid position 190. In place of the threonine codon (ACA) normally present in the luciferase gene at this site, each of the 3 possible nonsense codons (TAA, TAG, or TGA) and each of the 4 possible nucleotides (adenine, thymine, cytosine, or guanine) at the contextually important downstream +1 position following the nonsense codon are introduced by site-directed mutagenesis. As such, amino acid 190 in the luciferase gene containing a premature termination codon is either TAA, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com