Use of Modified Pyrimidine Compounds to Promote Stem Cell Migration and Proliferation
a technology of pyrimidine and pyrimidine, which is applied in the field of stimulating the proliferation and migration of mammalian stem cells, can solve the problems of motor deficits, significant hurdles that have yet to be substantially overcome in treatment, and the inability of the brain region to synthesize and release neurotransmitters vital to neuronal signaling, etc., and achieve the effect of efficient proliferation of stem cells and alleviation of neurological disorders
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Improvement of Cognitive Function in Aged Rat by the Transplantation of NSCs of the Invention
[0115]Human NSCs do not require any exogenous factors for differentiation and survived more than three weeks in basal media without the addition of any factor to support their survival (Qu et al., 2001, Neuroreport 12: 1127-32). Thus, it appears that human NSCs produce factors to differentiate and support themselves, which suggested that these cells could be transplanted into aged animals after treatment according to the methods of Apps. 1, 2 and the present invention.
[0116]Human NSCs, expanded without differentiation under the influence of mitogenic factors in supplemented serum-free media and pre-treated by the incorporation of bromodeoxyuridine (BrdU) into the nuclear DNA, were injected into the lateral ventricle of mature (6-month-old) and aged (24-month-old) rats. Human NSCs prepared according to the methods of the invention survived 30 days after xenotransplantation into aged rat brain...
example 2
Increase of Endogenous Stem Cell Proliferation by a Pyrimidine Derivative
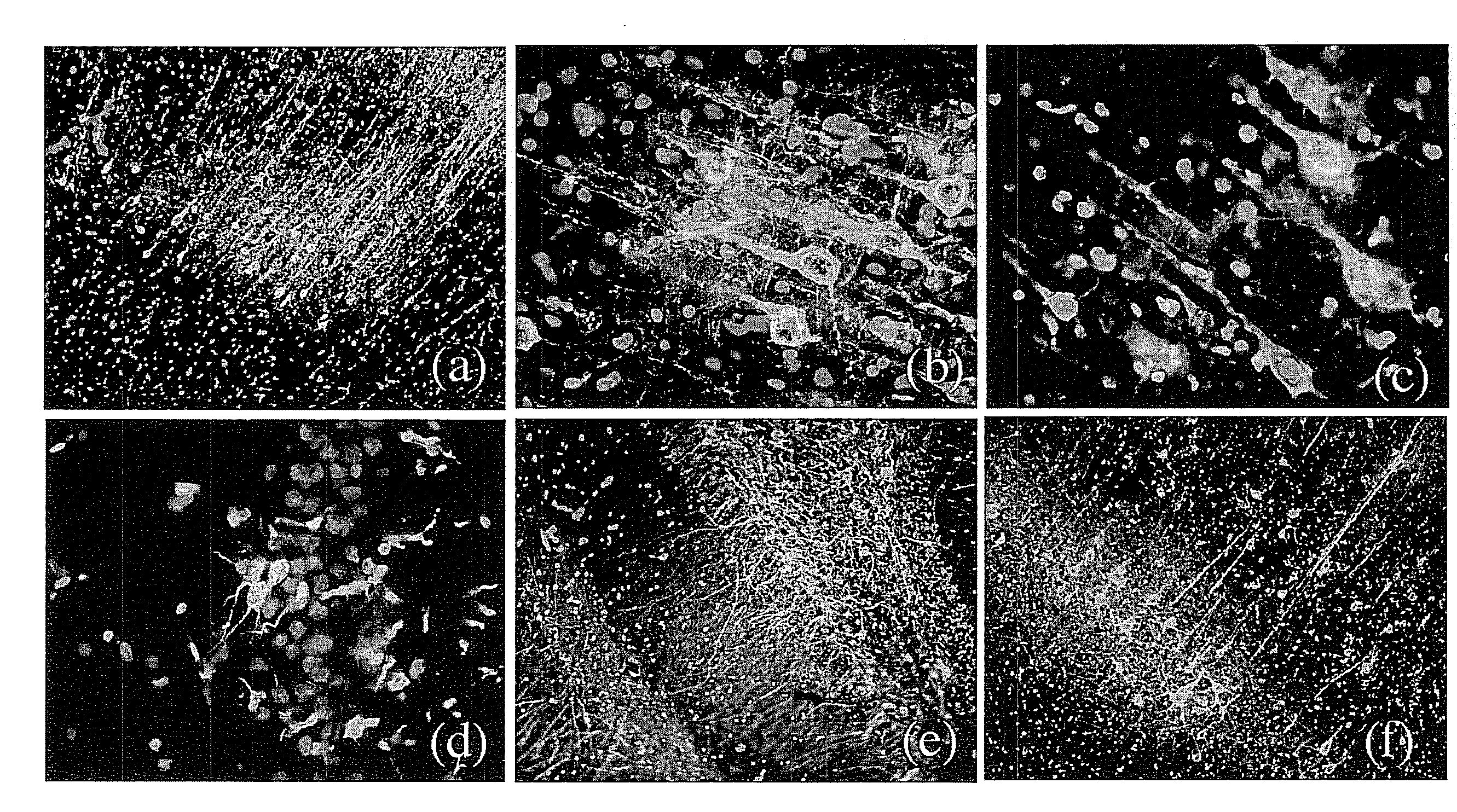
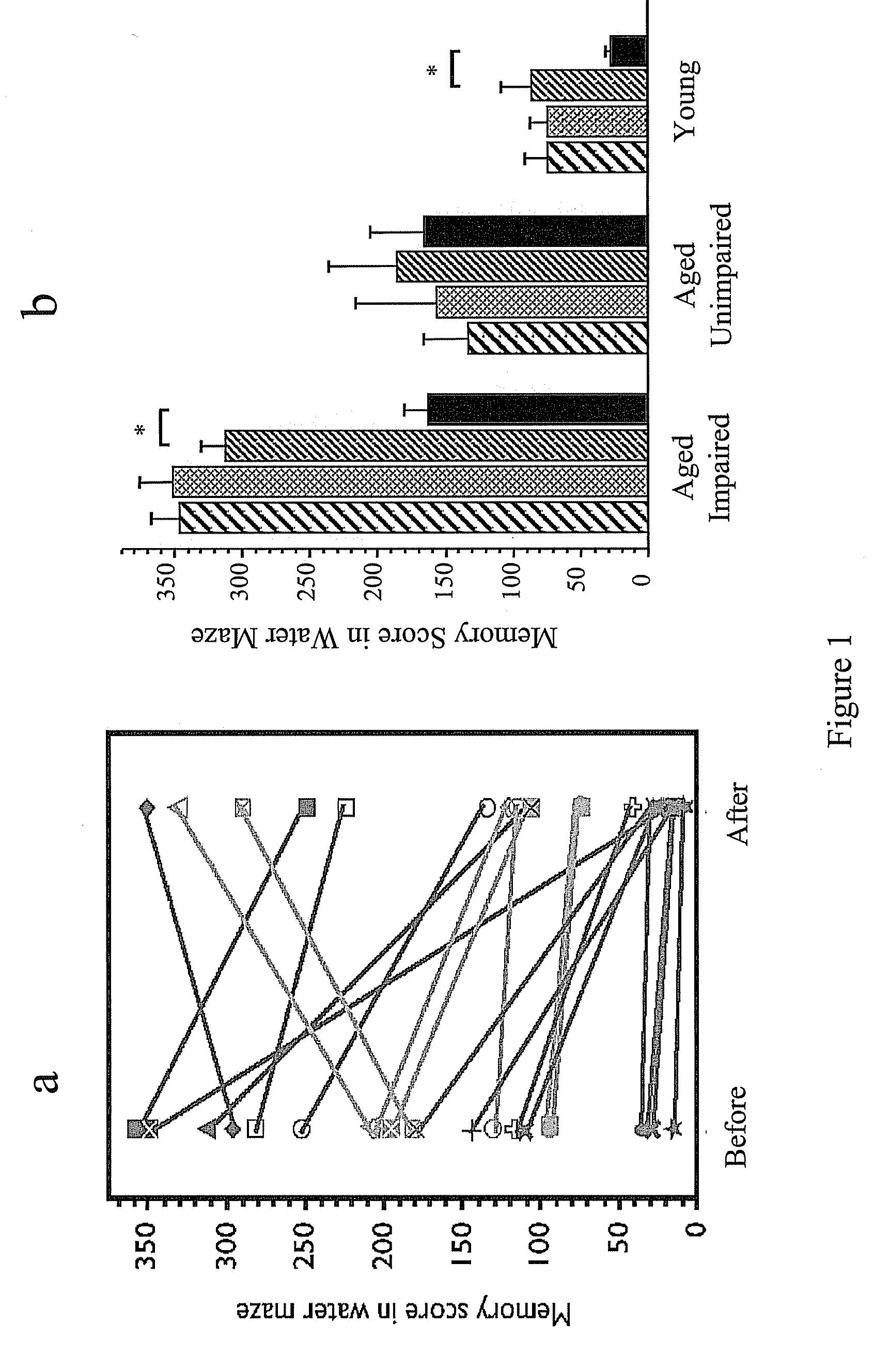
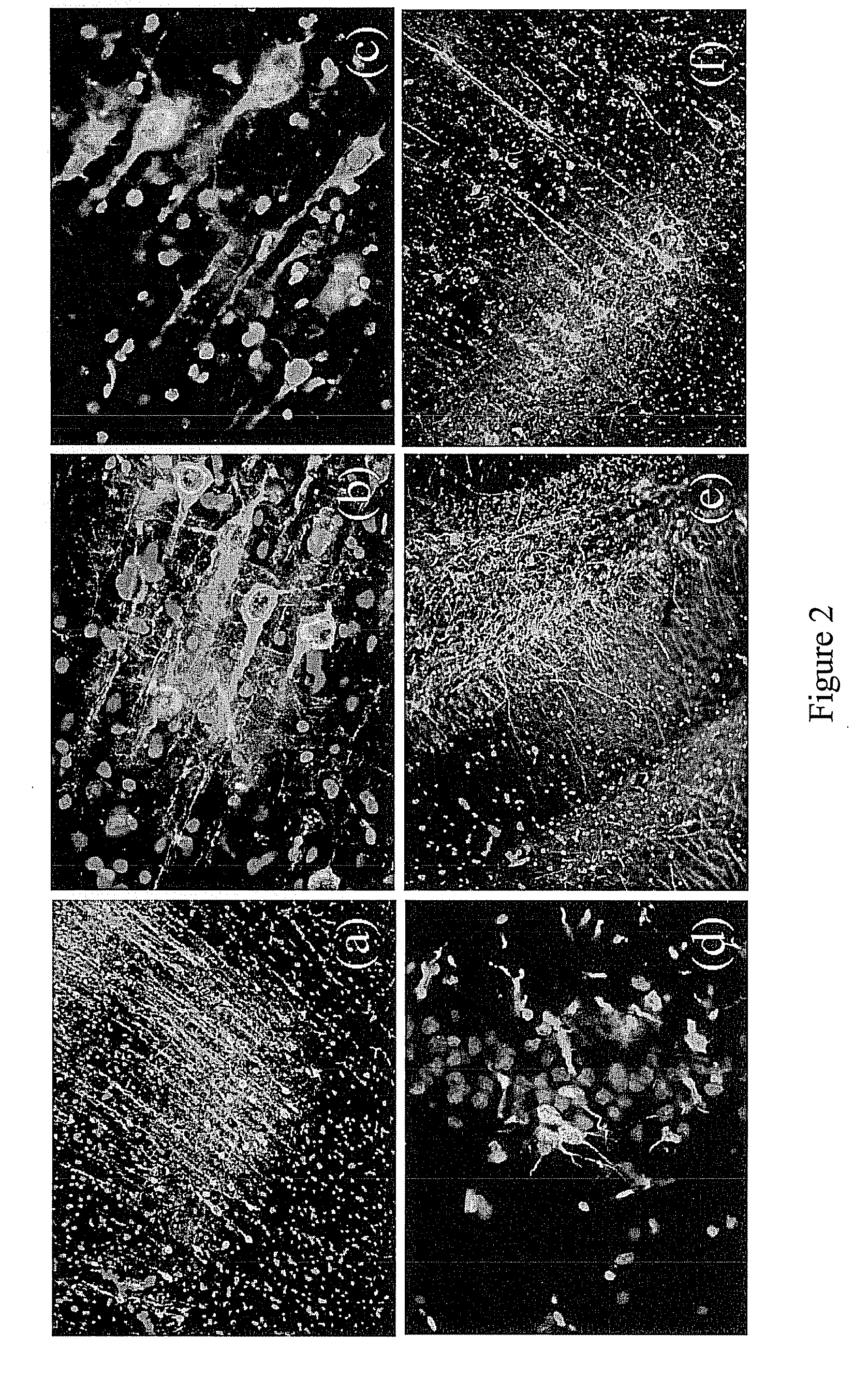
[0126]To investigate the effect of MS-818, a pyrimidine derivative, on stem cell population in vivo, MS-818 (3 mg / kg / day, i.p.) was injected for 5 days into aged (27-month old) male Fisher 344 rats. The same volume of saline was injected into control animals. Bromodeoxyuridine (BrdU) (100 mg / kg / day i.p.) was then injected for 3 days. Twenty-four hours after the last injection, the brains were removed and fixed for immunohistochemical detection of the proliferating cells by immunostaining for BrdU. The number of BrdU positive cells increased more than seven fold in the cerebral cortices of MS-8,8-treated animals compared to those of controls (FIG. 3a,b,e), indicating an increased neural stem cell population in the brain. In the area of the subventricular zone, a significant increase not only in the proliferation but also in the migration of stem cells was found (FIG. 3c,d). When this compound was injected direct...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com