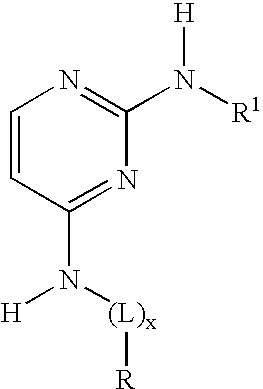
2-anilino-4-aminoalkyleneaminopyrimidines
a technology of aminopyrimidines and aminoalkylenes, applied in the field of aminoalkyleneaminopyrimidines, can solve the problems of symptomatic heart failure, reduced cardiac output, and impaired diastolic and/or systolic function, and achieve the effect of slowing the progression of heart failure, improving myocardial contraction and relaxation performan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
N2-(3-chlorophenyl)-N4-(3-(dimethylamino)propyl)pyrimidine-2,4-diamine (4)
[0179] Preparation of 2-(methylthio)pyrimidine-4(3H)-one (1): To a solution of sodium hydroxide (6.24 g, 156.07 mmol) in H2O (55 mL) at room temperature is added thiouridine (10 g, 78.03 mmol). The resulting mixture is stirred at room temperature for 20 min. Methyl iodide (5.45 mL, 87.40 mmol) in THF (10 mL) is added dropwise slowly and the mixture is stirred at room temperature for 18 hours. A white solid forms upon acidifying the mixture to pH 5 with glacial acetic acid. The mixture is allowed to stand at 0° C. (ice bath) for 2 hours and filtered to afford 7.4 g (67% yield) of the desired compound as a white solid. 1H NMR (DMSO-d6, 300 MHz): δ 2.45 (s, 3H), 6.07 (d, J=6.6 Hz, 1H), 7.85 (d, J=6.6 Hz, 1H).
[0180] Preparation of 2-(3-chlorophenylamino)pyrimidin-4(3H)-one (2): To 2-(methyl-thio)pyrimidine-4(3H)-one, 1, (4.88 g, 34.37 mmol) in diglyme (20 mL) is added 3-chloroaniline (4.3 mL, 68.74 mmol). The re...
example 2
N4-[3-(dimethylamino)-2,2-dimethylpropyl]-N2-[3-(trifluoromethyl)phenyl]pyrimidine-2,4-diamine hydrochloride (7)
[0223] Preparation of 2-(3-trifluoromethylphenylamino)pyrimidin-4(3H)-one (5): To 2-(methyl-thio)pyrimidine-4(3H)-one, 1, (5 g, 35.21 mmol) in diglyme (25 mL) is added 3-trifluoromethylaniline (5.26 mL, 42.25 mmol). The resulting mixture is heated to reflux and stirred for 18 hours. A solid forms upon cooling the mixture to room temperature. The solid is washed with hexanes to afford 1.9 g (21% yield) of the desired compound. MS (ESI, pos. ion) m / z: 256 (M+1).
[0224] Preparation of 4-chloro-N-(3-trifluoromethylphenyl)pyrimidin-2-amine (6): To 2-(3-chloro-phenylamino)pyrimidin-4(3H)-one, 5, (1.9 g, 7.4 mmol) and N,N-dimethyl-aniline (2 mL) is added of phosphorus oxychloride (20 mL). The resulting mixture is heated to reflux for 15 minutes, cooled to room temperature and concentrated in vacuo. The residue is neutralized to pH 7 with 1M NaOH (aqueous). The organic layer is e...
example 3
N2-(3-chlorophenyl)-N4-(4-(dimethylamino)butyl)pyrimidine-2,4-diamine (8)
[0227] Preparation of N2-(3-chlorophenyl)-N4-(4-(dimethylamino)butyl)pyrimidine-2,4-diamine(8): To 4-chloro-N-(3-chlorophenyl)pyrimidin-2-amine (0.2 g, 0.84 mmol) in THF (4 mL) is added diisopropylethylamine (0.29 mL, 1.67 mmol) followed by 4-dimethylamino)butylamine (0.2 g, 1.67 mmol). The resulting mixture is heated to reflux for 6 hours. Another 2 equivalents of 4-dimethylamino)butylamine (0.2 g, 1.67 mmol) are added and the reaction heated at reflux for 18 hours. The reaction is cooled to room temperature and concentrated in vacuo. The residue is diluted with water (5 mL) and extracted with EtOAc (3×25 mL). The combined organic layers are dried (MgSO4) and concentrated in vacuo. This residue is purified over silica (5% MeOH in CH2Cl2 with 0.7% Et3N) to afford 7 mg (3% yield) of the desired compound. 1H NMR (CD3OD, 300 MHz): δ 1.62-1.66 (m, 4H), 2.26 (s, 6H), 2.39-2.44 (m, 2H), 3.46-3.48 (m, 2H), 5.97 (d, J...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com