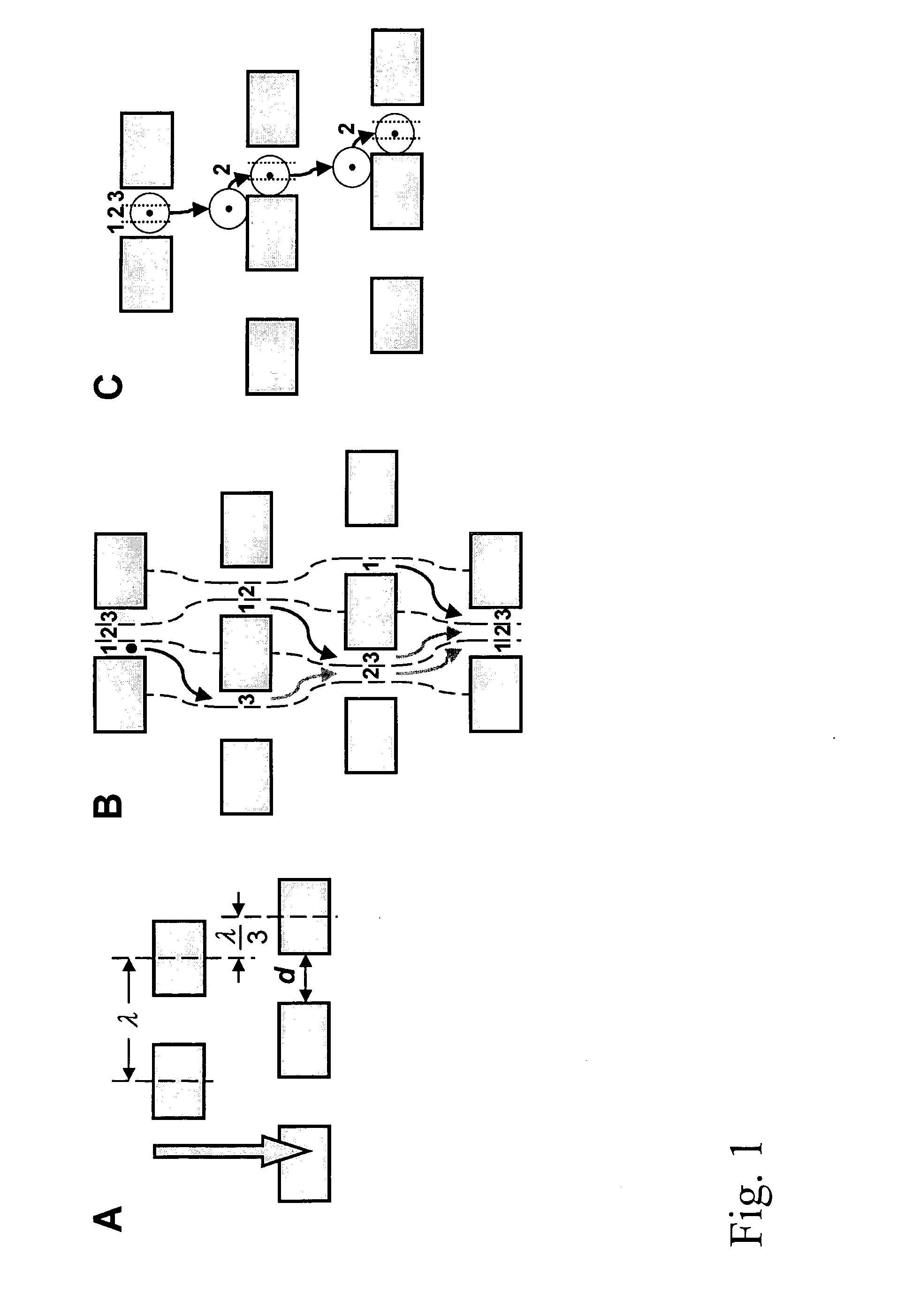
Devices and methods for enrichment and alteration of cells and other particles
a technology of cell and particle, applied in the field of cell separation, medical diagnostics, microfluidic devices, can solve the problems of large volume of sample and skilled operators, low quantities, and large equipment costs, and achieve the effects of reducing the hydrodynamic size of a particle, increasing deformation, and decreasing volum
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
A Silicon Device Multiplexing 14 3-stage Array Duplexes
[0241]FIGS. 42A-42E show an exemplary device, characterized as follows.
[0242] Dimension: 90 mm×34 mm×1 mm
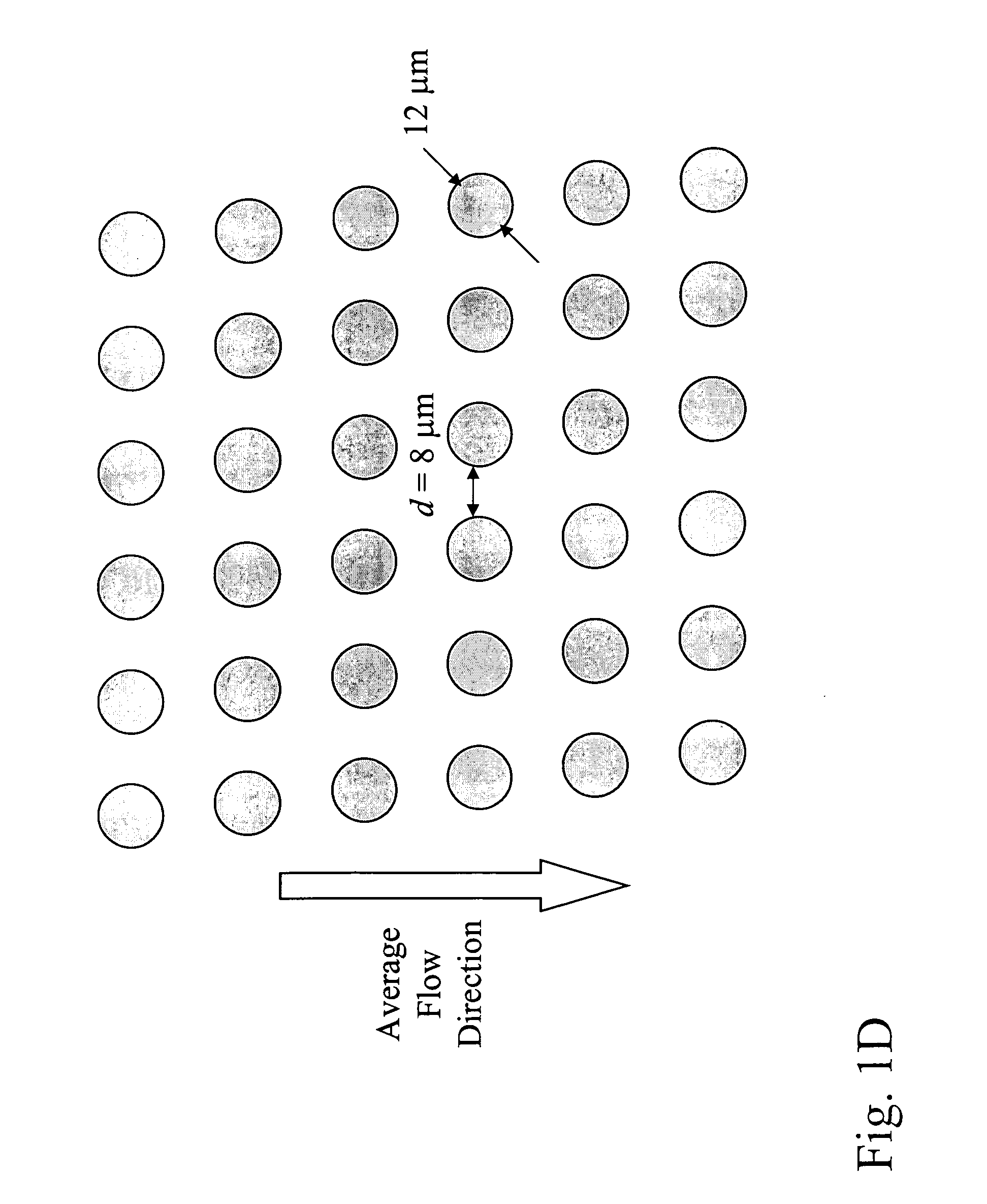
[0243] Array design: 3 stages, gap size=18, 12 and 8 μm for the first, second and third stage, respectively. Bifurcation ratio=1 / 10. Duplex; single bypass channel
[0244] Device design: multiplexing 14 array duplexes; flow resistors for flow stability
[0245] Device fabrication: The arrays and channels were fabricated in silicon using standard photolithography and deep silicon reactive etching techniques. The etch depth is 150 μm. Through holes for fluid access are made using KOH wet etching. The silicon substrate was sealed on the etched face to form enclosed fluidic channels using a blood compatible pressure sensitive adhesive (9795, 3M, St Paul, Minn.).
[0246] Device Packaging: The device was mechanically mated to a plastic manifold with external fluidic reservoirs to deliver blood and buffer to the device and extract the...
example 2
A Silicon Device Multiplexing 14 Single-Stage Array Duplexes
[0251]FIGS. 44A-44D show an exemplary device, characterized as follows.
[0252] Dimension: 90 mm×34 mm×1 mm
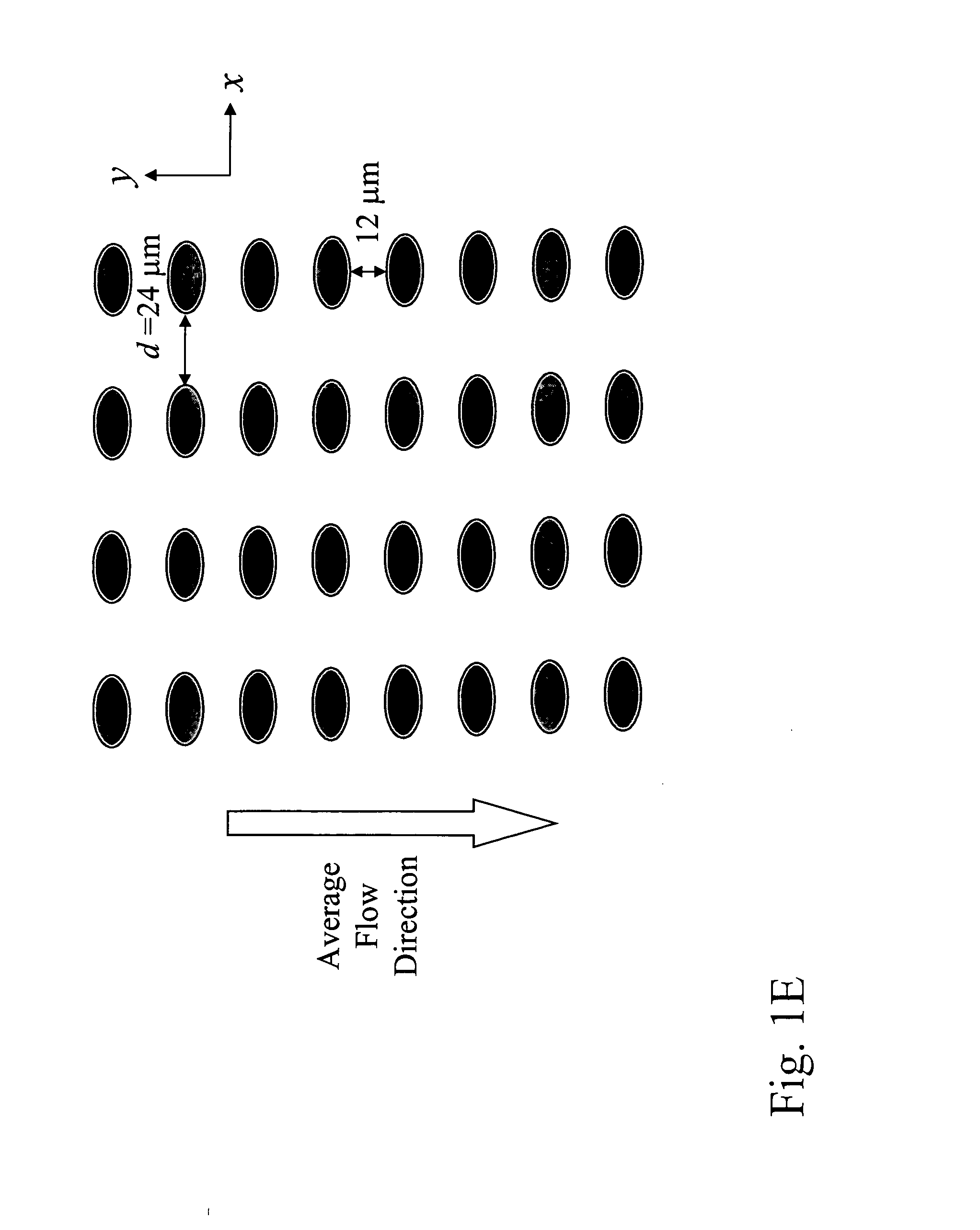
[0253] Array design: 1 stage, gap size=24 μm. Bifurcation ratio=1 / 60. Duplex; double bypass channel
[0254] Device design: multiplexing 14 array duplexes; flow resistors for flow stability
[0255] Device fabrication: The arrays and channels were fabricated in silicon using standard photolithography and deep silicon reactive etching techniques. The etch depth is 150 μm. Through holes for fluid access are made using KOH wet etching. The silicon substrate was sealed on the etched face to form enclosed fluidic channels using a blood compatible pressure sensitive adhesive (9795, 3M, St Paul, Minn.).
[0256] Device Packaging: The device was mechanically mated to a plastic manifold with external fluidic reservoirs to deliver blood and buffer to the device and extract the generated fractions.
[0257] Device Operation: An external...
example 3
Separation of Fetal Cord Blood
[0261]FIG. 45 shows a schematic of the device used to separate nucleated cells from fetal cord blood.
[0262] Dimension: 100 mm×28 mm×1 mm
[0263] Array design: 3 stages, gap size=18, 12 and 8 μm for the first, second and third stage, respectively. Bifurcation ratio 1 / 10. Duplex; single bypass channel.
[0264] Device design: multiplexing 10 array duplexes; flow resistors for flow stability.
[0265] Device fabrication: The arrays and channels were fabricated in silicon using standard photolithography and deep silicon reactive etching techniques. The etch depth is 140 μm. Through holes for fluid access are made using KOH wet etching. The silicon substrate was sealed on the etched face to form enclosed fluidic channels using a blood compatible pressure sensitive adhesive (9795, 3M, St Paul, Minn.).
[0266] Device Packaging: The device was mechanically mated to a plastic manifold with external fluidic reservoirs to deliver blood and buffer to the device and ext...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thick | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com