Method for treatment of macular degeneration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiment 50
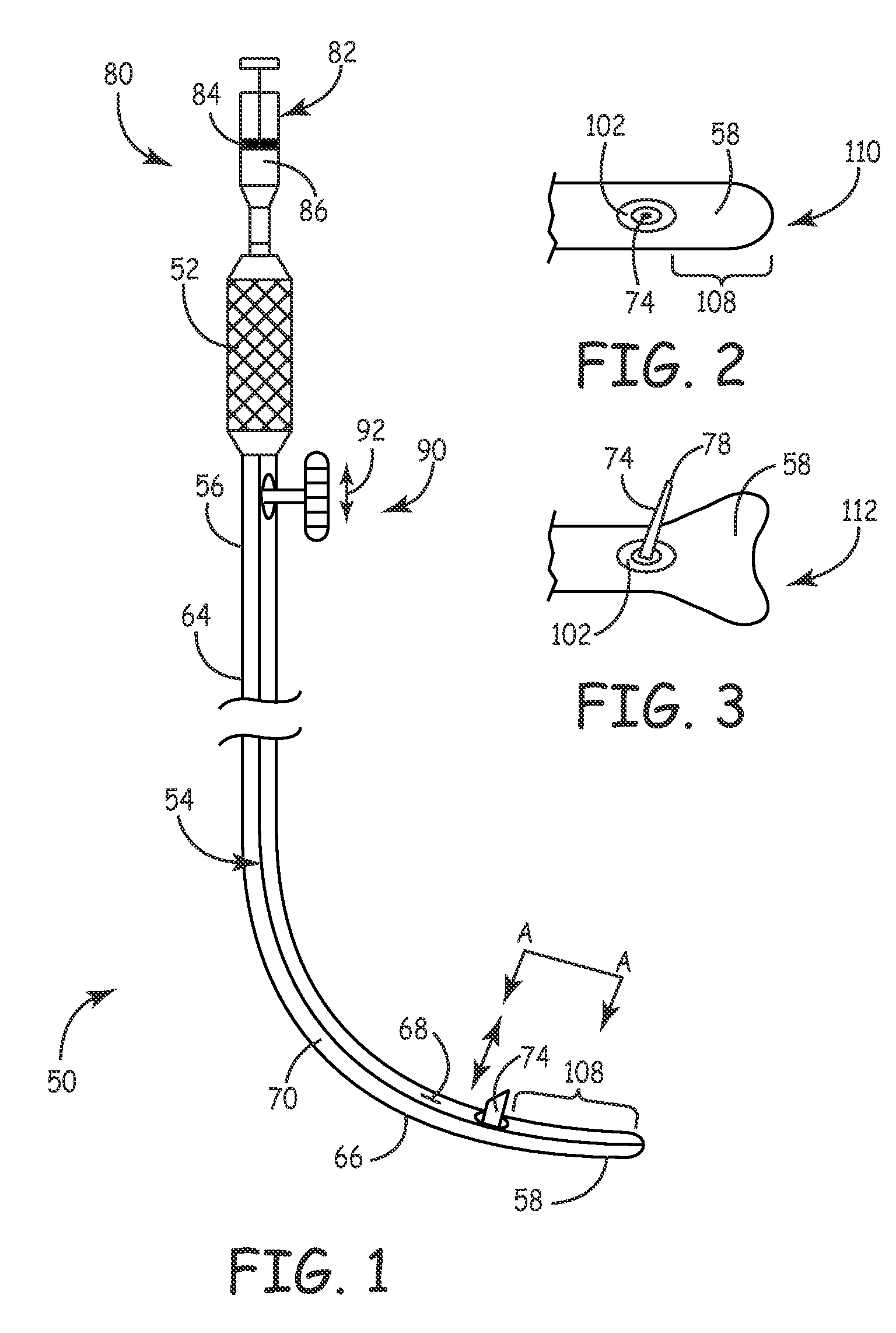
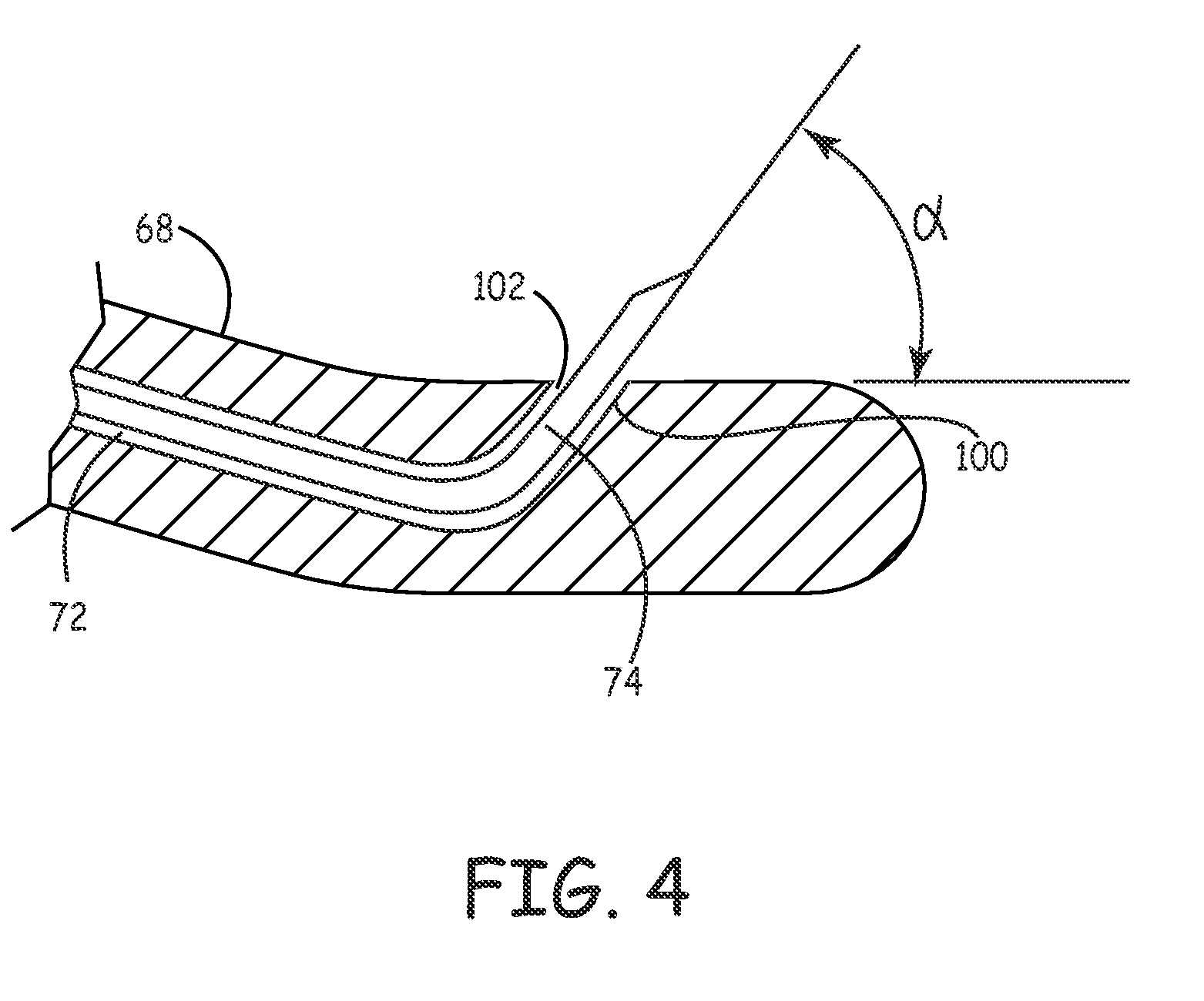
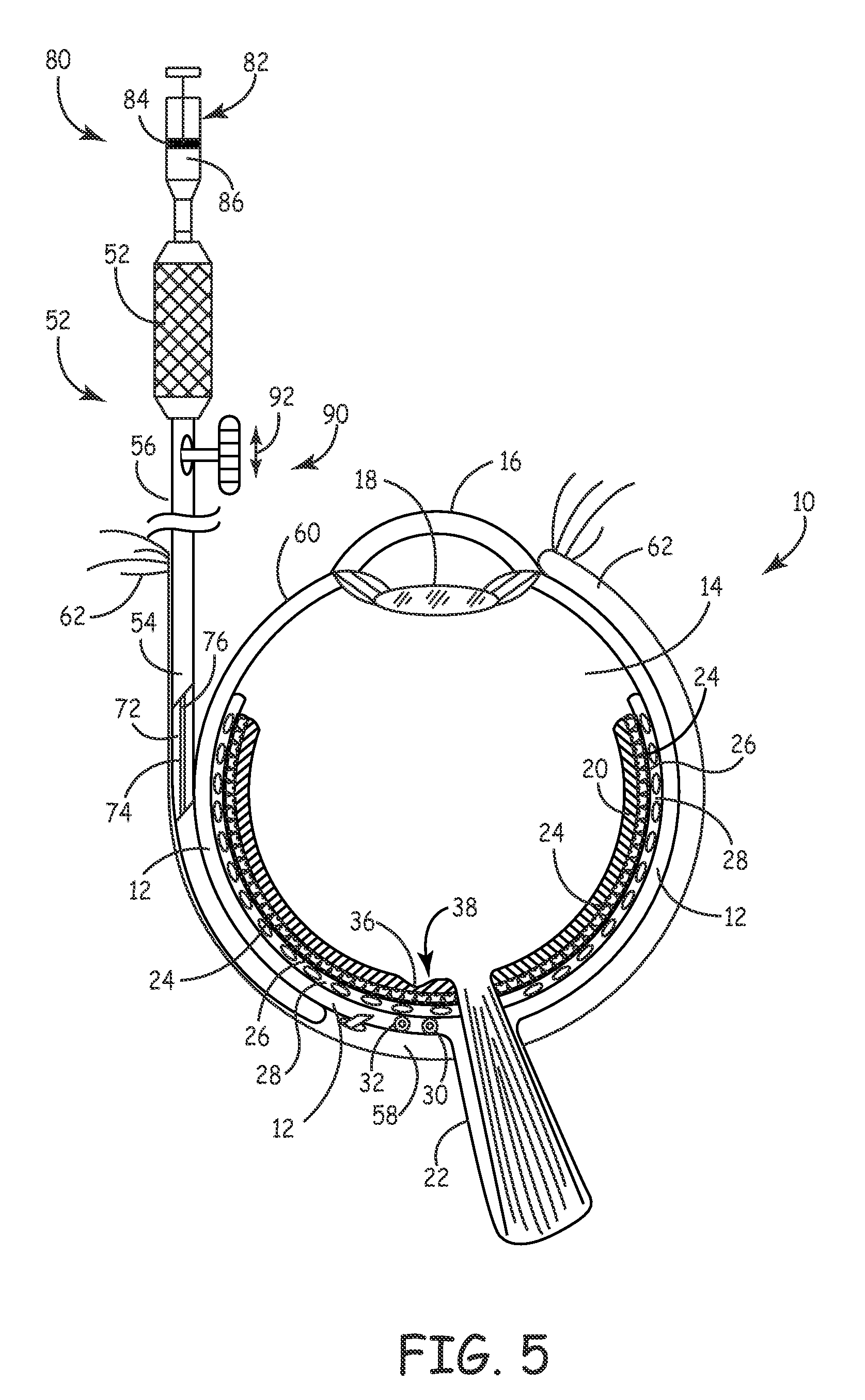
[0053] An embodiment 50 for providing macular degeneration therapy in accordance with the present invention is schematically shown in FIGS. 1-6 generally, with FIGS. 5-6 illustrating the invention relative to an eye. System 50 includes a handle 52 and a elongated hollow probe 54 with a proximal end 56 and a distal end 58. Probe 54 preferably is curved to negotiate the curved surface of the eyeball 60 and is sized to fit between a patient's sclera 12 and the eye lid 62.
[0054] More specifically, probe 54 includes a portion 64 and a curved probe portion 66. The curved probe portion is configured to conform to the curvature of an eye 10, thus reducing the likelihood of bruising or exertion of undue force on an eye during a procedure. The radius of curvature of the curved probe portion 66 may be in the range of about 12 mm to about 13 mm. Portion 66 may further include an eye-conforming and engaging surface 68 to further ease stresses on an eye during a procedure. It will be understood t...
embodiment 120
[0064] In FIGS. 7-9 another embodiment of an apparatus for drug delivery into sclera 12 is shown. In this embodiment 120 of the present invention the single needle 74 of apparatus 50 is replaced by a micro-needle array 122. Thus, referring to FIG. 7, embodiment 120 includes a probe 124 having a passage 126. Housed within the passage 126 is a tube 128 that is fluidly connected to the reservoir 80, which provides a solution of therapeutic agents such as lipase to the micro-needle array 122. Micro-needle array 122 comprises a plurality of needles 130 fluidly connected to tube 128.
[0065] The micro-needle array 122 may assume one of two possible positions: a retracted, inactive position and an extended or advanced, active position in which the micro-needles 130 of the array 122 penetrate the sclera 12. To move the array 122 between the inactive and active positions, a spring 132 may be provided. Spring 132 is attached to an advancement mechanism such as mechanism 90. The array 122 may be...
embodiment 160
[0069] In FIG. 11 another embodiment 160 of the present invention is shown. This embodiment is similar to apparatus 150 depicted in FIG. 10, but enhances delivery of the therapeutic agents into the sclera with iontophoresis. Thus, apparatus 160 includes a probe 162 having a passage 164 that houses a tube 166 connected to a reservoir 80 and one or more diffusion and conductive pads. As illustrated, each of the pads are both conductive and serve as diffusion pads, though separate pads could be provided for each purpose if desired. Thus, the apparatus 160 also includes one or more porous conductive pads 170 and 172 soaked with therapeutic agents and electrically connected to a negative electrode by wire 174 and one or more conductive pads 168 electrically connected to a positive electrode by wire 176. That is, the conductive pads are connected to positive and negative electrodes with wires 174 and 176 of a DC power source, not shown in the figure. Electric current passing between the p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Therapeutic | aaaaa | aaaaa |
| Proximity effect | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com