Stereoselective antifibrillogenic peptides and peptidomimetics thereof
a technology of peptides and peptides, applied in the direction of peptide/protein ingredients, carrier-bound/immobilised peptides, nervous disorders, etc., can solve the problems of inability to significantly dissolve in situ amyloidotic fibrils, toxic to surrounding cells once deposited, and not known, widely accepted therapies or treatments that significantly dissolve amyloidotic fibrils, etc., to reduce the formation of fibrils, increase the number of amyloidotic fibrils, and reduce the immunogenicity of fibrils
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
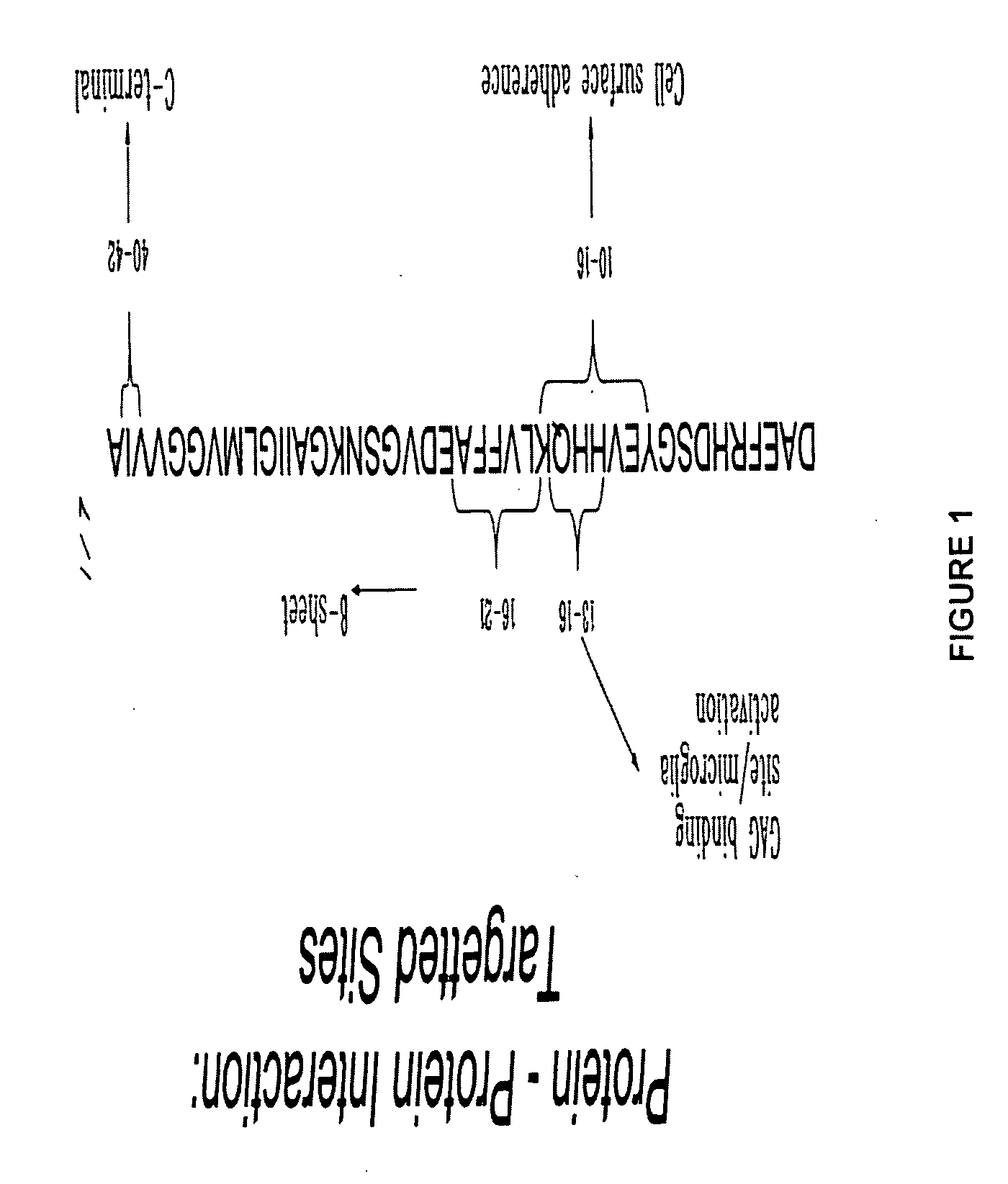
[0054] As illustrated in FIG. 1, internal regions of the Aβ sequence have been shown to confer characteristics of the amyloid protein. Indeed, the region between amino acid 13-16 (His-His-Gln-Lys, SEQ ID NO:23) of the amyloid protein is responsible for the interaction between the Aβ protein and the glycosaminoglycan moiety of the proteoglycans (Kisilevsky, R., et al., Proteoglycans and amyloid fibrillogenesis: The nature and origin of amyloid fibrils, Wiley, Chichester (CIBA Foundation Symposium 1997), pp. 58-72). Proteoglycans are known to promote amyloid fibril formation as well as protect these fibrils from proteolysis (Gupta-Bansal, R., et al., 1995, The Journal of Biological Chemistry, 270:18666-18671). More recently, the same region has been determined to play a role in the activation process of microglial cells by Aβ (Giulian, D., et al., 1998, The Journal of Biological Chemistry, 273(45):29719-29726). This 13-16 region of Aβ, often referred to as the GAG binding site, is als...
PUM
| Property | Measurement | Unit |
|---|---|---|
| infrared spectra | aaaaa | aaaaa |
| hydrophobic | aaaaa | aaaaa |
| min-width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com