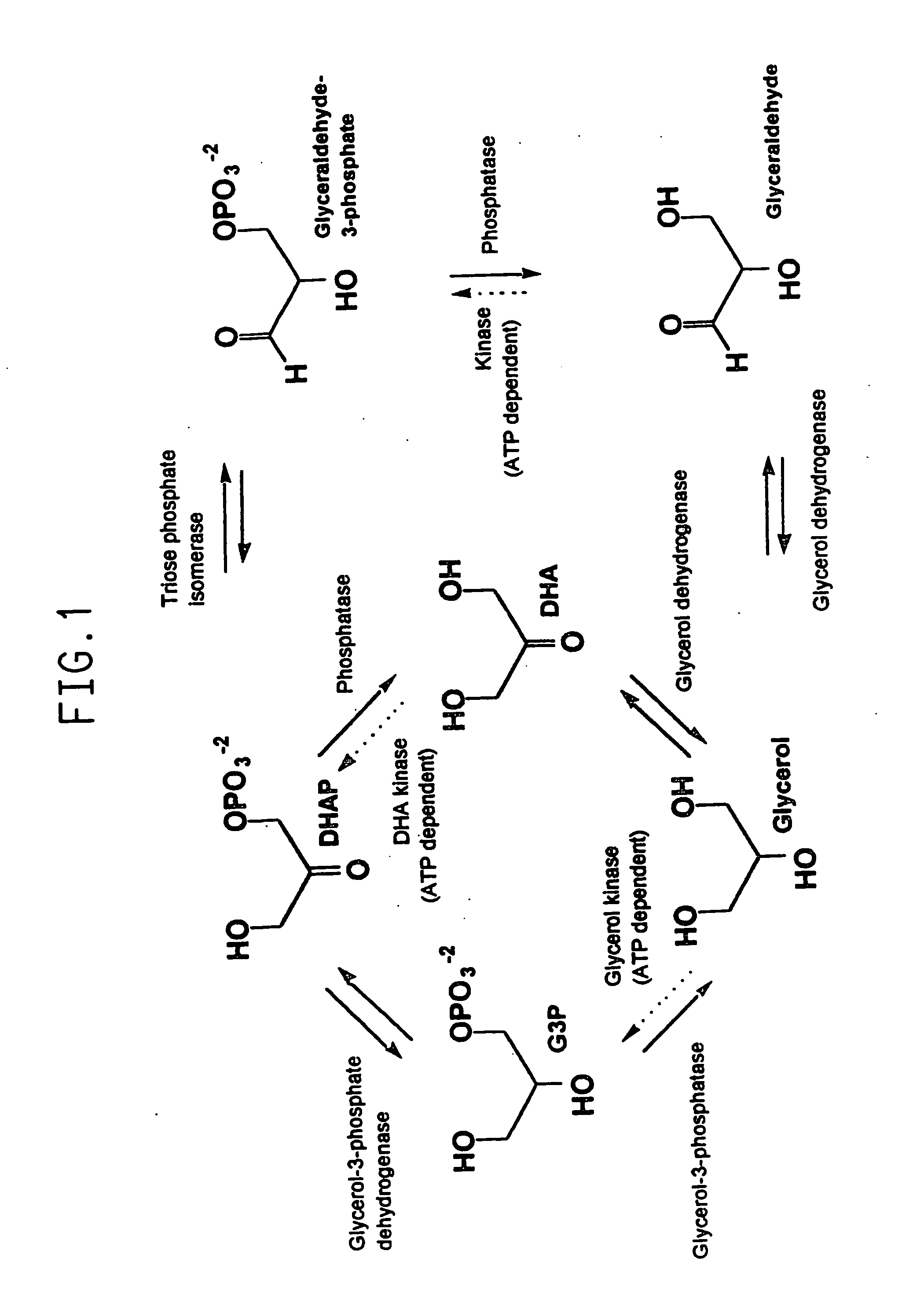
Method for the production of glycerol by recombinant organisms
a recombinant organism and glycerol technology, applied in the field of molecular biology and the use of recombinant organisms, can solve the problems of laborious and inefficient processes, limited methods, and often subject to the need to control osmotic stress
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Production of Glycerol from E. coli Transformed with G3PDH Gene
Media
[0113] Synthetic media was used for anaerobic or aerobic production of glycerol using E. coli cells transformed with pDAR1A. The media contained per liter 6.0 g Na2HPO4, 3.0 g KH2PO4, 1.0 g NH4Cl, 0.5 g NaCl, 1 mL 20% MgSO40.7H2O, 8.0 g glucose, 40 mg casamino acids, 0.5 ml 1% thiamine hydrochloride, 100 mg ampicillin.
Growth Conditions
[0114] Strain AA200 harboring pDAR1A or the pTrc99A vector was grown in aerobic conditions in 50 mL of media shaking at 250 rpm in 250 mL flasks at 37° C. At A600 0.2-0.3 isopropylthio-β-D-galactoside was added to a final concentration of 1 mM and incubation continued for 48 h. For anaerobic growth samples of induced cells were used to fill Falcon #2054 tubes which were capped and gently mixed by rotation at 37° C. for 48 h. Glycerol production was determined by HPLC analysis of the culture supernatants. Strain pDAR1A / AA200 produced 0.38 g / L glycerol after 48 h under anaerobic co...
example 2
Production of Glycerol from E. coli Transformed with G3P Phosphatase Gene (GPP2)
Media
[0115] Synthetic phoA media was used in shake flasks to demonstrate the increase of glycerol by GPP2 expression in E. coli. The phoA medium contained per liter: Amisoy, 12 g; ammonium sulfate, 0.62 g; MOPS, 10.5 g; Na-citrate, 1.2 g; NaOH (1 M), 10 mL; 1 M MgSO4, 12 mL; 100× trace elements, 12 mL; 50% glucose, 10 mL; 1% thiamine, 10 mL; 100 mg / mL L-proline, 10 mL; 2.5 mM FeCl3, 5 mL; mixed phosphates buffer, 2 mL (5 mL 0.2 M NaH2PO4+9 mL 0.2 M K2HPO4), and pH to 7.0. The 100× traces elements for phoA medium / L contained: ZnSO4.7H2O, 0.58 g; MnSO4.H2O, 0.34 g; CuSO4.5H2O, 0.49 g; CoCl2.6H2O, 0.47 g; H3BO3, 0.12 g, NaMoO4.2H2O, 0.48 g.
Shake Flasks Experiments
[0116] The strains pAH21 / DH5α (containing GPP2 gene) and pPHOX2 / DH5α (control) were grown in 45 mL of media (phoA media, 50 ug / mL carbenicillin, and 1 ug / mL vitamin B12) in a 250 mL shake flask at 37° C. The cultures were grown under aerobic ...
example 3
Production of Glycerol from D-Glucose Using Recombinant E. coli Containing Both GPP2 and DAR1
[0117] Growth for demonstration of increased glycerol production by E. coli DH5α-containing pAH43 proceeds aerobically at 37° C. in shake-flask cultures (erlenmeyer flasks, liquid volume ⅕th of total volume).
[0118] Cultures in minimal media / 1% glucose shake-flasks are started by inoculation from overnight LB / 1% glucose culture with antibiotic selection. Minimal media are: filter-sterilized defined media, final pH 6.8 (HCl), contained per liter: 12.6 g (NH4)2SO4, 13.7 g K2HPO4, 0.2 g yeast extract (Difco), 1 g NaHCO3, 5 mg vitamin B12, 5 mL Modified Balch's Trace-Element Solution (the composition of which can be found in Methods for General and Molecular Bacteriology (P. Gerhardt et al., eds, p. 158, American Society for Microbiology, Washington, D.C. (1994)). The shake-flasks are incubated at 37° C. with vigorous shaking for overnight, after which they are sampled for GC analysis of the su...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelengths | aaaaa | aaaaa |
| wavelengths | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com