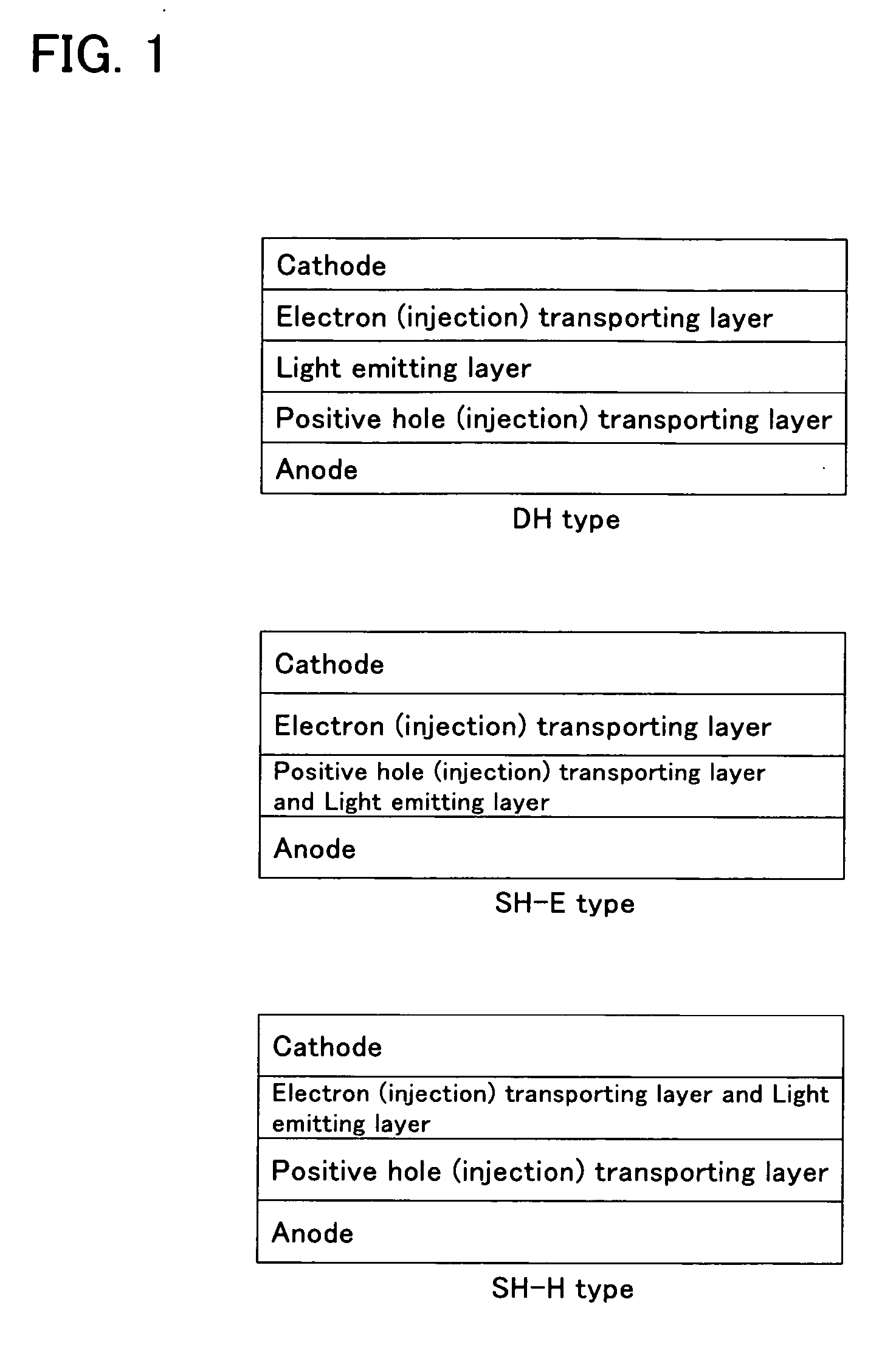
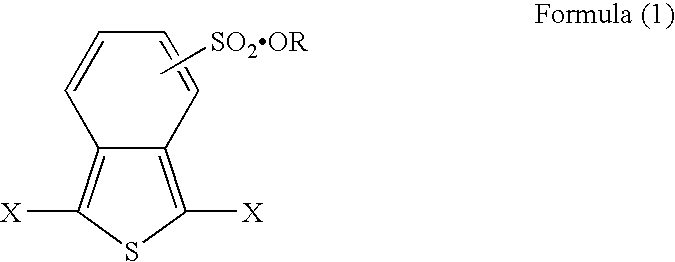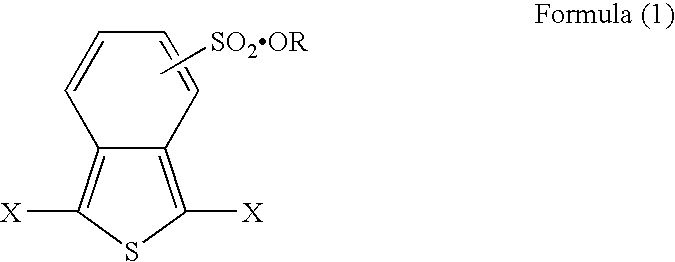Thiophene derivative and organic electroluminescent element
a technology of thiophene and derivative, which is applied in the direction of discharge tube luminescnet screen, thermoelectric device, natural mineral layered product, etc., can solve the problems of unfavorable light emission efficiency, unsatisfactory light emission efficiency, and significant difficulty in introducing a sulfonic group to all polybenzo[c]thiophene units, etc., to achieve excellent light emission efficiency and water resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0036] 5.27 parts of dibromo-o-toluene was heated to reflux in 70 parts of toluene in the presence of phosphorus trisulfide for 8 hours to obtain benzo[c]thiophene. 0.86 parts of thus obtained benzo[c]thiophene was heated to reflux with 0.56 parts of cuprous bromide in dehydrated methylene chloride for 5 hours to obtain 0.47 parts of dibromobenzothiophene. The bromide was sulfonated by fuming sulfuric acid. The sulfide was heated to reflux with an excessive amount of dibutyl sulfate in dehydrated tetrahydrofuran for 12 hours. Then, an unreacted product and impurity were removed, dried under reduced pressure, and thus obtained a thiophene derivative of the present invention represented by the formula 1a (brown powder, decomposition temperature: 157° C. (elimination of a dibutyl group), melting point: 270° C.):
[0037] The results of elementary analysis and NMR analysis of the compound represented by the formula 1a are as follows.
[Elementary Analysis]
[0038] Calculated value C=33.7, ...
examples 2 to 4
[0042] In Example 2, in the same manner as in Example 1 except that dimethyl sulfate was used instead of dibutyl sulfate, a thiophene derivative 1b of the present invention in which the butyl group represented by the formula 1a was a methyl group was obtained.
[0043] In Example 3, in the same manner as in Example 1 except that diethyl sulfate was used instead of dibutyl sulfate, a thiophene derivative 1c of the present invention in which the butyl group represented by the formula 1a was an ethyl group was obtained.
[0044] In Example 4, in the same manner as in Example 1 except that dipropyl sulfate was used instead of dibutyl sulfate, a thiophene derivative id of the present invention in which the butyl group represented by the formula 1a was a propyl group was obtained.
[0045] The analysis results are shown in Table 1.
TABLE 1EstereliminationMeltingElementary analysisCom-temperaturepoint(measured value)pound(° C.)(° C.)CHSOExample 21b149-15125527.81.416.511.9Example 31c15325031.12...
example 5
[0046] 4.3 parts of the thiophene derivative represented by the formula 1a obtained in Example 1 and 2.4 parts of 2, 5-dibromothiophene were heated to reflux in dimethyl formamide in the presence of nickel chloride and triphenyl sulfone for 4 hours, thus obtained 0.7 parts of a copolymer represented by the following formula 2a, wherein a weight average molecular weight (measured by GPC, polystyrene standard) of the obtained copolymer obtained was 20,000 and the copolymer was soluble to toluene by 1 mass % or more:
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com