Transdifferentiation of glial cells
a technology of glial cells and glial cells, which is applied in the direction of nervous system cells, biochemical apparatus and processes, biocide, etc., can solve the problems of unsuitable use of these cells, difficult use of this process for producing cells for human implantation, immunological rejection and potential contamination, etc., and achieves rapid shipping to medical facilities. , the effect of avoiding the need for glial cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
6.0 EXAMPLE 1
Analysis of Astrocytes Treated with bFGF
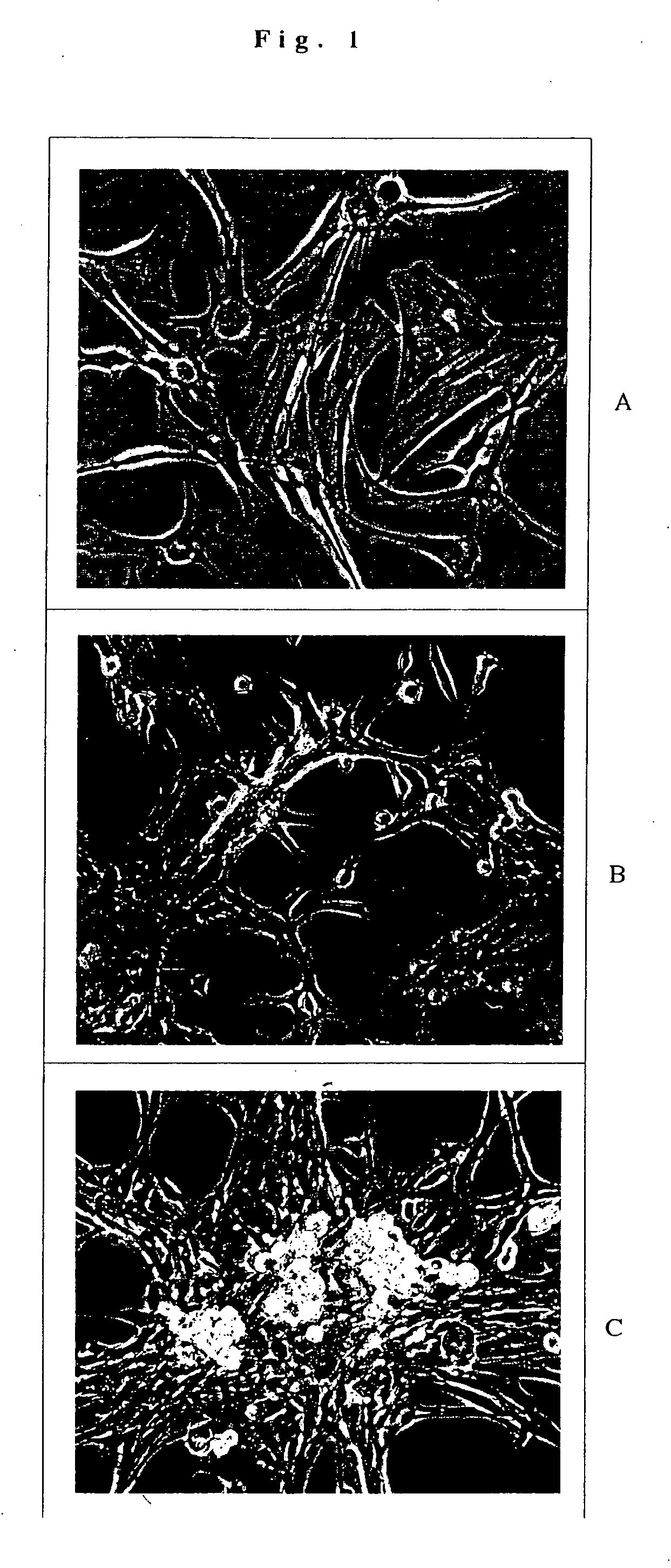
[0043] A series of experiments are grouped together in this example to show conditions for the culture of cells and treatments for transdifferentiation.
6.1 In Vitro Culture of Astrocytes
6.1.1 In Vitro Culture of Astrocytes from Human Neural Progenitor Cell Derived Astrocytes
[0044] Astrocytes derived from human neural stem cells were grown on plastic tissue culture flasks in the presence of 10% FCS in DMEM / F-12 culture medium. The media was changed twice per week. After confluency, the cells were trypsinized (0.25% trypsin for 2 minutes at 37 degrees C.), treated with trypsin neutralization solution (CALBIOCHEM) and the cells were pelleted, resuspended and plated at a ratio of 1:3. In general, it was important to passage the cells at no more than 1:5 ratio; further, at a 1:2 ratio confluency was typically achieved in 7-10 days.
6.1.2 In Vitro Culture of Mammalian Astrocytes from Mammalian Nervous Tissue
[0045] Nervous tissue...
example 2
7.0 EXAMPLE 2
Treatment with bFGF without a Dissociation Step
[0052] The cells were treated as described in Example 1 except as described below, most notably in the omission of the trypsinization step. In brief, monolayers of astrocytes were washed once in DMEM / F-12 and defined medium containing DMEM / F-12 and SUPPLEMENT B27 (GIBCO), 20-ng per ml bFGF (PEPROTECH), and 1 microgram per ml heparin (SIGMA) was added for 2 and 5 days. The cells were then grown in Medium I for 7 days.
[0053] Immunochemistry revealed that there were no MAP2ab or Beta-tubulin III positive cells in either the 2-day or 5-day groups, indicating that no neurons were present. Therefore cell dissociation enhances the production of multipotent cells because cells that underwent the same treatment plus a dissociation step were made multipotent and then differentiated into neurons.
example 3
8.0 EXAMPLE 3
Pretreatment and Treatment of Cells with bFGF
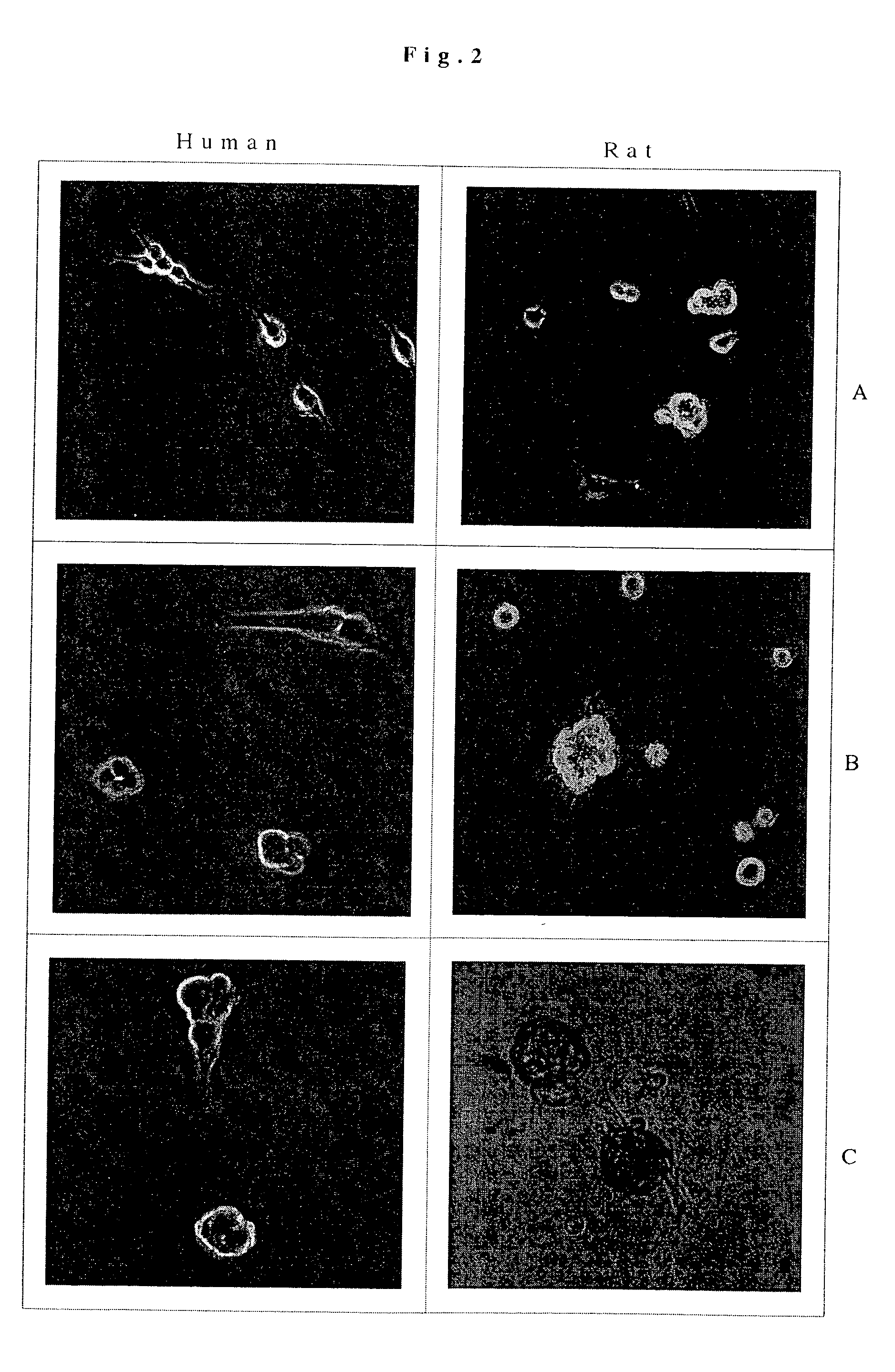
[0054] These experiments were performed as described in Example 1 except for the differences described herein; these results are the results of multiple experiments. Cultured astrocytes were dissociated and plated on tissue culture plastic. The cells were cultured with 20 ng per ml bFGF plus heparin for three to six weeks. The cells were then placed in Medium I, Medium II, or Medium III for 7 and 14 days on laminin-coated substrates.
[0055] After 7 days in Medium I, II, or III, 60%-80% of the cells were GFAP positive astrocytes. Up to 30% of the cells were Beta-tubulin III positive, GFAP negative neurons (FIG. 3A) and 5-10% of the cells were MAP2ab positive. In Media II and III, 0.5%-2.0% cells were positive for the oligodendrocyte markers CNPase and O4. Cells in Medium II marked for CNPase and O4 were more strongly positive for these markers than cells in the other media. In Medium I oligodendrocytes were mainly absent. The...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com