Vascular endothelial cell growth factor antagonists and uses thereof
a technology of endothelial cells and growth factors, which is applied in the field of vascular endothelial cell growth factor antagonists, can solve the problems of increased endothelial cell leakage in skin, retina, tumor tissues, etc., and achieves the effects of improving the survival rate of endothelial cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Anti- hVEGF Monoclonal Antibodies
[0109] To obtain hVEGF conjugated to keyhole limpet hemocyanin (KLH) for immunization, recombinant hVEGF (165 amino acids), Leung, et al., Science 246:1306 (1989), was mixed with KLH at a 4:1 ratio in the presence of 0.05% glutaraldehyde and the mixture was incubated at room temperature for 3 hours with gentle stirring. The mixture then was dialyzed against phosphate buffered saline (PBS) at 4ΕC. overnight.
[0110] Balb / c mice were immunized four times every two weeks by intraperitoneal injections with 5:g of hVEGF conjugated to 20:g of KLH, and were boosted with the same dose of hVEGF conjugated to KLH four days prior to cell fusion.
[0111] Spleen cells from the immunized mice were fused with P3X63Ag8U.1myeloma cells, Yelton, et al., Curr. Top. Microbiol. Immunol. 81:1 (1978), using 35% polyethylene glycol (PEG) as described. Yarmush, et al., Proc. Nat. Acad. Sci. 77:2899 (1980). Hybridomas were selected in HAT medium.
[0112] Supernat...
example 2
Characterization Of Anti-hVEGF Monoclonal Antibodies
A. Antigen Specificity
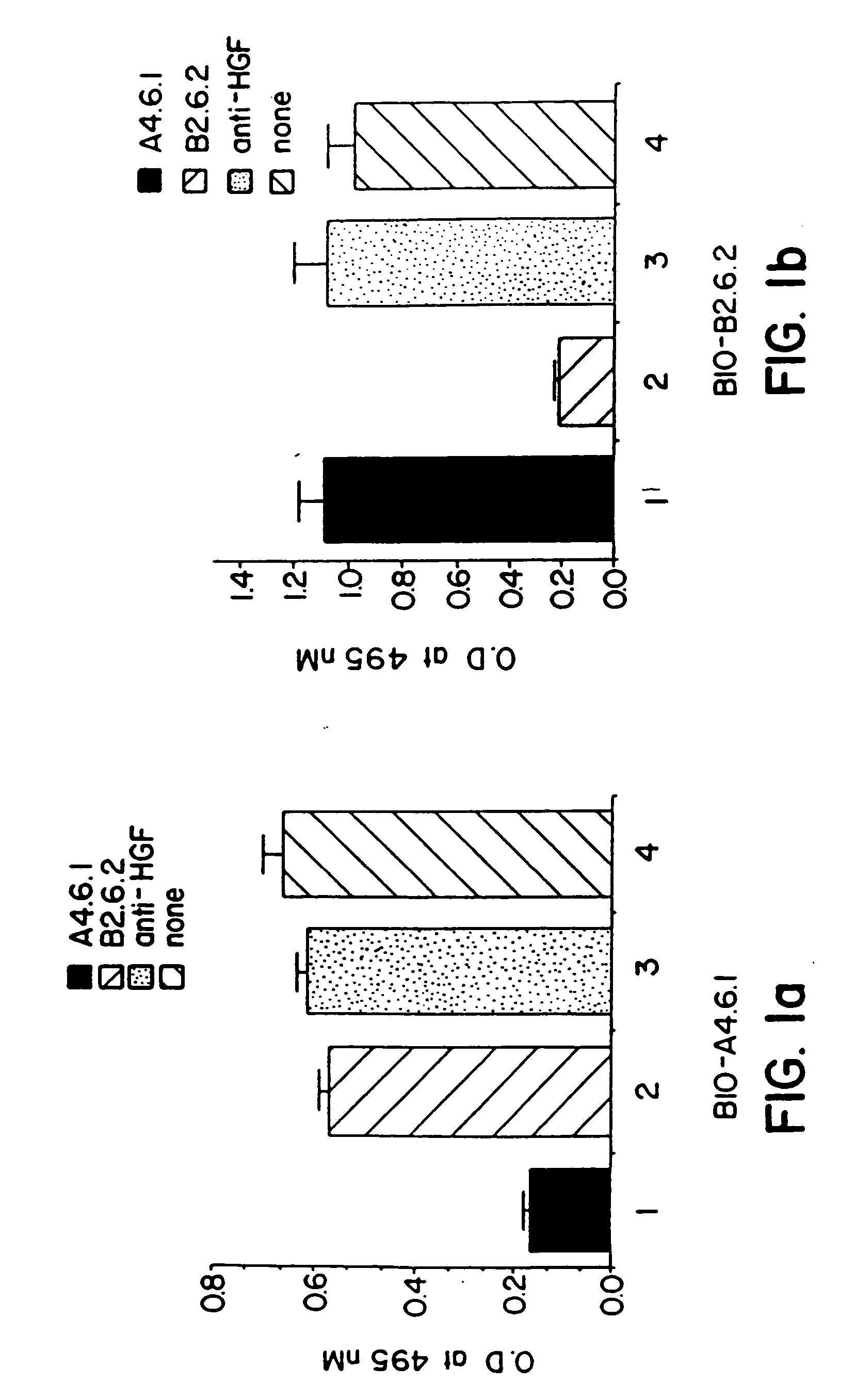
[0113] The binding specificities of the anti-hVEGF monoclonal antibodies produced by the A4.6.1 and B2.6.2 hybridomas were determined by ELISA. The monoclonal antibodies were added to the wells of microtiter plates that previously had been coated with hVEGF, FGF, HGF, or epidermal growth factor (EGF). Bound antibody was detected with peroxidase conjugated goat anti-mouse IgG immunoglobulins. The results of those assays confirmed that the monoclonal antibodies produced by the A4.6.1 and B2.6.2 hybridomas bind to hVEGF, but not detectably to those other protein growth factors.
B. Epitope Mapping
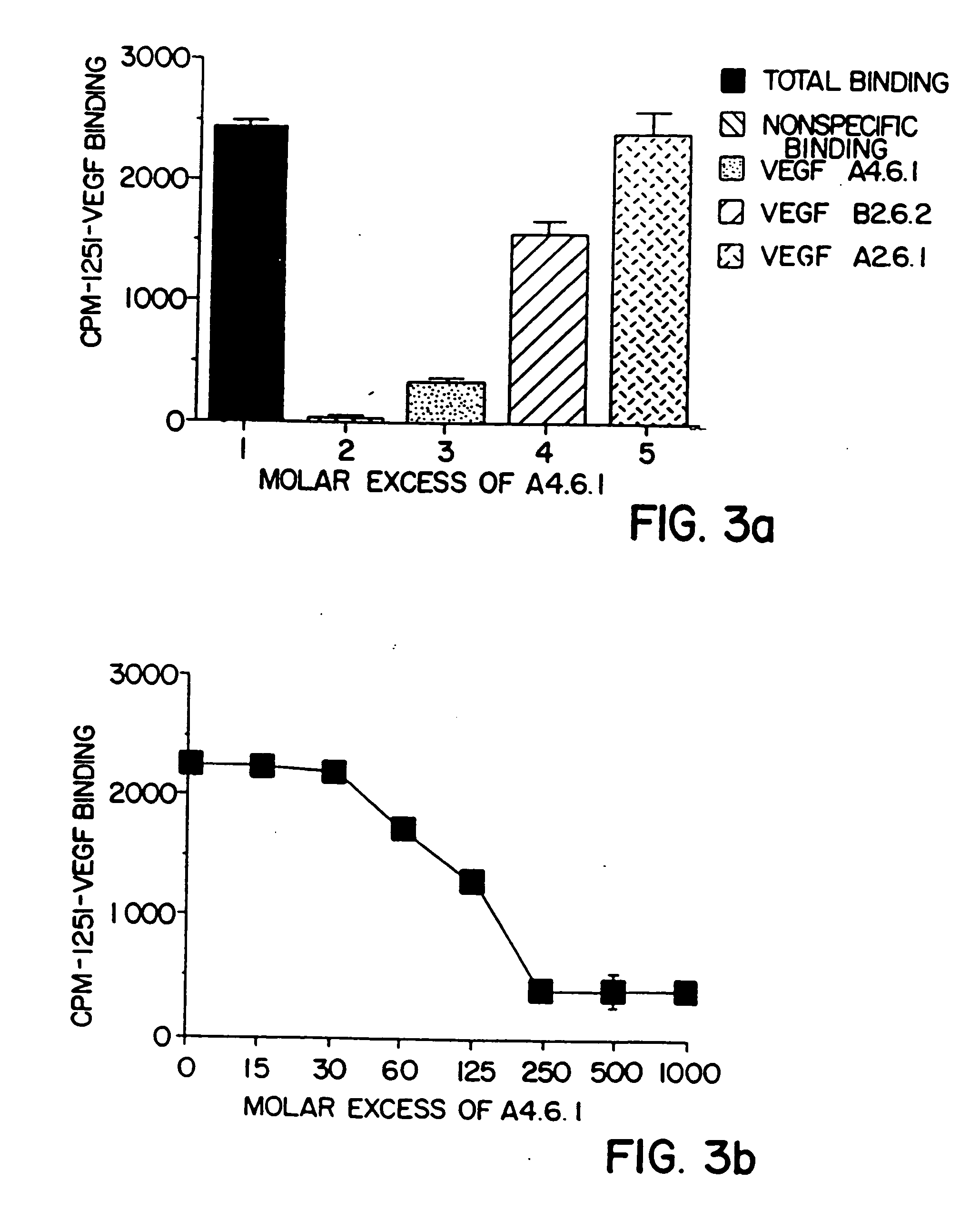
[0114] A competitive binding ELISA was used to determine whether the monoclonal antibodies produced by the A4.6.1 and B2.6.2 hybridomas bind to the same or different epitopes (sites) within hVEGF. Kim, et al., Infect. Immun. 57:944 (1989). Individual unlabeled anti-hVEGF monoclonal antibodies (A4.6.1 or B2.6.2) or ir...
example 3
Preparation Of VEGF Receptor—IgG Fusion Proteins
A.
[0130] The nucleotide and amino acid coding sequences of the flt hVEGF receptor are disclosed in Shibuya, et al., Oncogene 5:519-524 (1990). The coding sequence of the entire extracellular domain of the flt hVEGF receptor was fused to the coding sequence of human IgGl heavy chain in a two-step process.
[0131] Site-directed mutagenesis was used to introduce a BstBI restriction into DNA encoding flt at a site 5′ to the codon for amino acid 759 of flt, and to convert the unique BstEII restriction site in plasmid pBSSK−FC, Bennett, et al., J. Biol. Chem. 266:23060-23067 (1991), to a BstBI site. The modified plasmid was digested with EcoRI and BstBI and the resulting large fragment of plasmid DNA was ligated together with an EcoRI-BstBI fragment of the flt DNA encoding the extracellular domain (amino acids 1-758) of the flt hVEGF receptor.
[0132] The resulting construct was digested with ClaI and NotI to generate an approximately 3.3 kb...
PUM
| Property | Measurement | Unit |
|---|---|---|
| apparent molecular mass | aaaaa | aaaaa |
| apparent molecular weight | aaaaa | aaaaa |
| apparent molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com