Methods and apparatus for gel-free qualitative and quantitative proteome analysis, and uses therefore
a proteome and qualitative and quantitative technology, applied in biochemistry apparatus and processes, peptide preparation methods, component separation, etc., can solve the problems of difficult analysis using this method, high labor intensity, and sequential 2d-pages, and achieve the effects of high acidity or basic protein, high labor intensity, and high labor intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Specific Chemical Alteration of Methionine-Residues
[0190] A protein peptide mixture was generated according to the method described in the invention and the relatively rare amino acid methionine was selected for alteration. As documented in the literature, one approach to alter methionine is by chemical oxidation, which can lead to sulfoxide-formation and to sulfone-formation. Peptides comprising methionine can be converted into their sulfone derivatives by using strong oxidizing conditions such as with performic acid or other per-acids (Toennies and Homiller, 1942 and Hirs, 1956). It is known that the stronger oxidizing conditions are rather harsh and not selective enough. The formation of methionine-sulfoxide proceeds upon contact of methionine with the air. However, in the presence of 0.5% H2O2 at room temperature and low pH (1% TFA), this reaction is completed in less than 30 minutes. Interestingly, under these mild conditions, it was observed that both cysteine and tryptophan,...
example 2
Specific Chemical Alteration of Cysteine-Residues
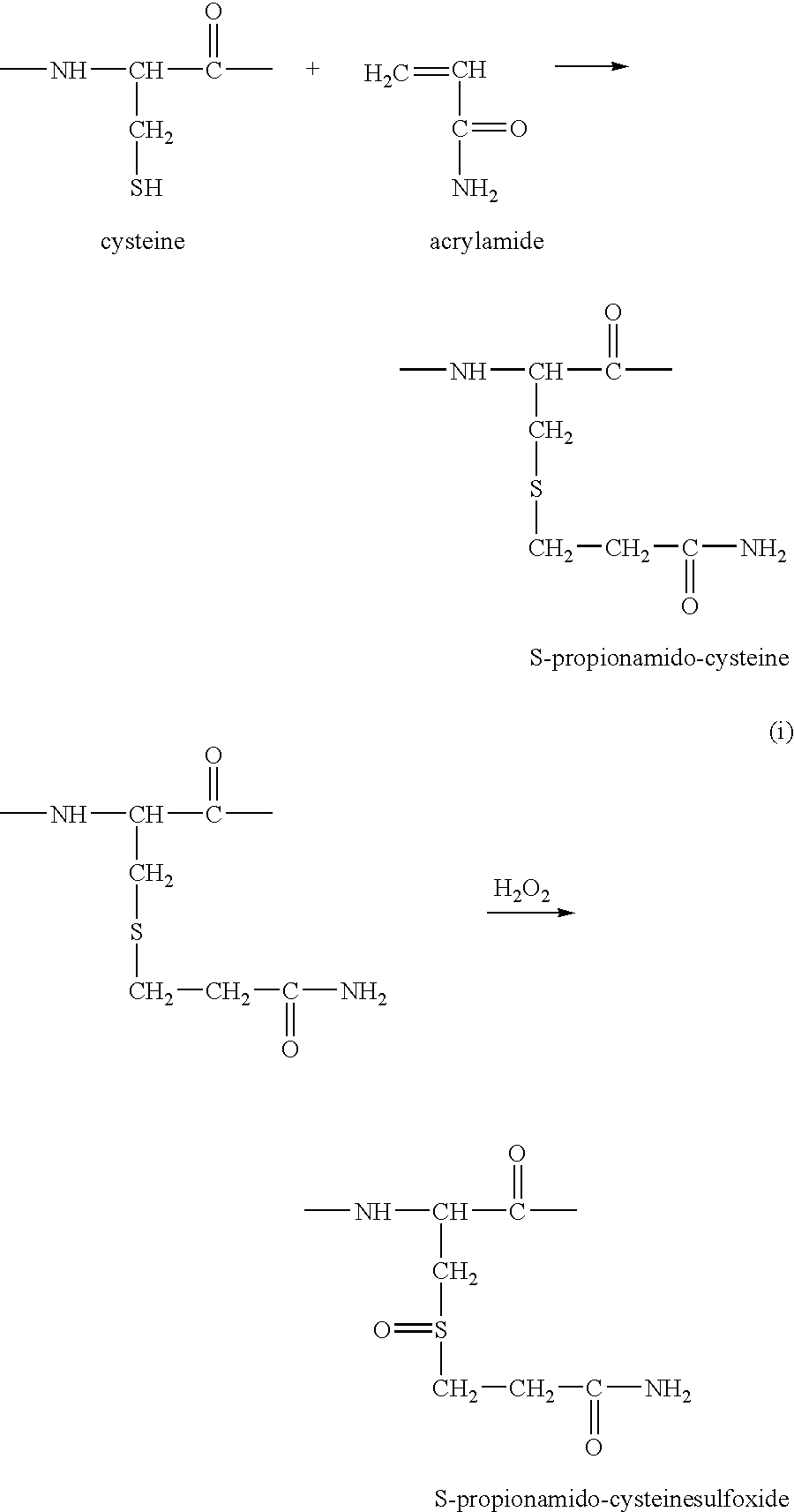
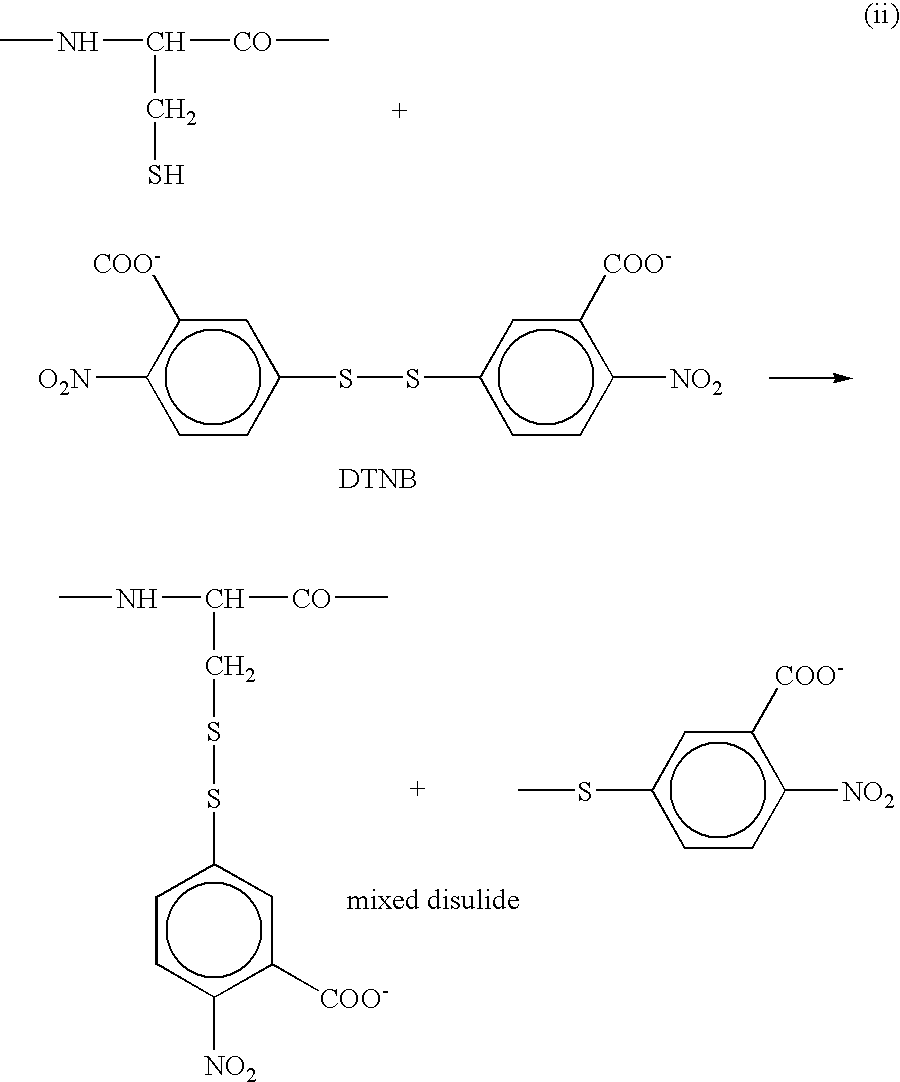
[0192] A protein peptide mixture is generated with one of the methods described herein before and a specific chemical alteration of cysteine residues is carried out. Said alteration is for instance based on the specific conversion of cysteine peptides into a more hydrophilic derivative, which undergoes a hydrophilic shift during reversed phase HPLC. Several reagents can fulfill these requirements. For instance, reactions with iodoacetamide, iodoacetate, ethyleneimine, bromoethylamine, acrylamide and 4-vinyl pyridine, all convert cysteine into compounds that behave more hydrophilic in reverse phase-conditions. In addition these compounds all undergo oxidation by H2O2 resulting in the formation of their corresponding sulfoxide derivatives, which are even more hydrophilic. It is important to mention that the shift due to oxidation is less pronounced here than in the case of the methionine oxidation. However, when combining the shifts be...
example 3
Specific Chemical Alteration of the Sum of Methionine and Cysteine Residues
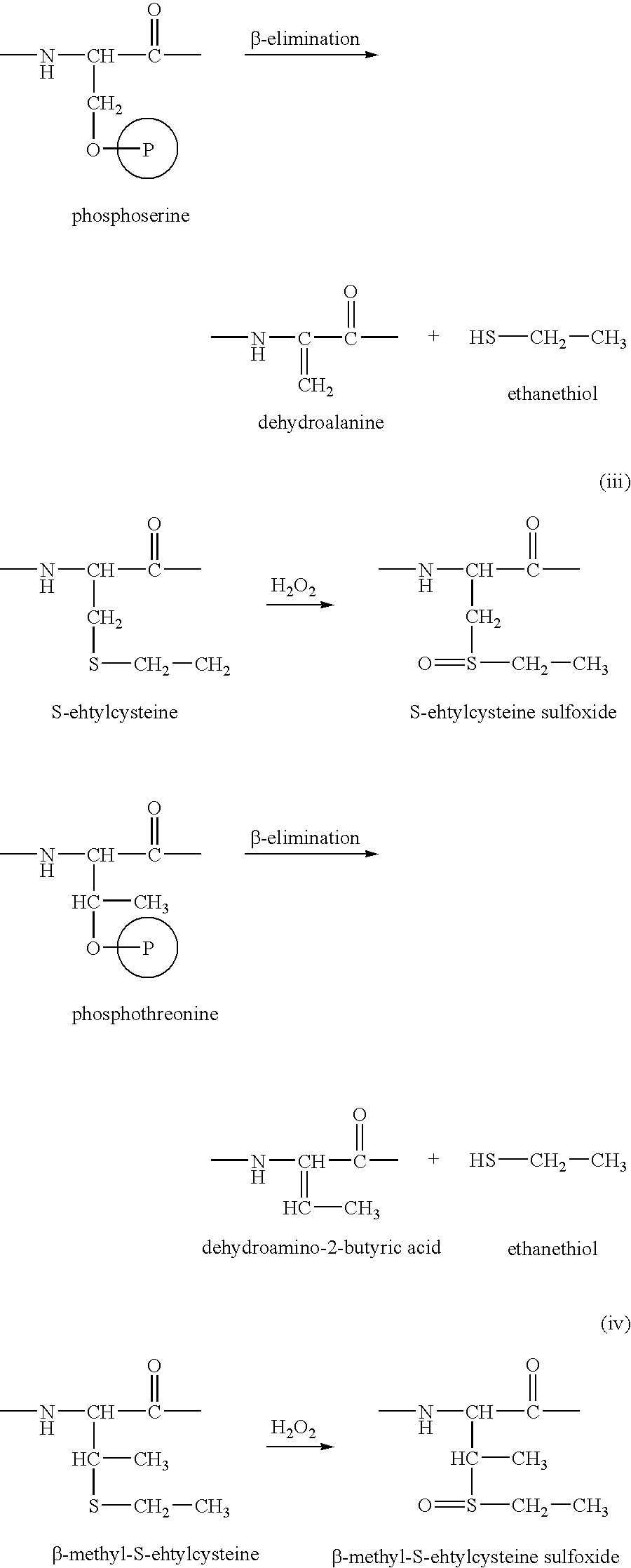
[0197] The procedure is identical to the procedure for specifically altering cysteine-residues (see Example 2) with the exception that the pre-oxidation step of the methionine residues is omitted. The reaction sequence starts with the reduction of a protein mixture with tributyl phosphine, followed by enzymatic or chemical cleavage. The protein peptide mixture is separated by RP-HPLC and each fraction is altered by reaction with acrylamide, immediately followed by oxidation with H2O2. The methionine-peptides are now oxidized together with the altered cysteine peptides and both types of flagged peptides show a hydrophilic shift when chromatographically separated using similar conditions as in the primary run. FIG. 5 summarizes the various reaction sequences in the Met-sorting, Cys-sorting and Met+Cys-sorting modes.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com