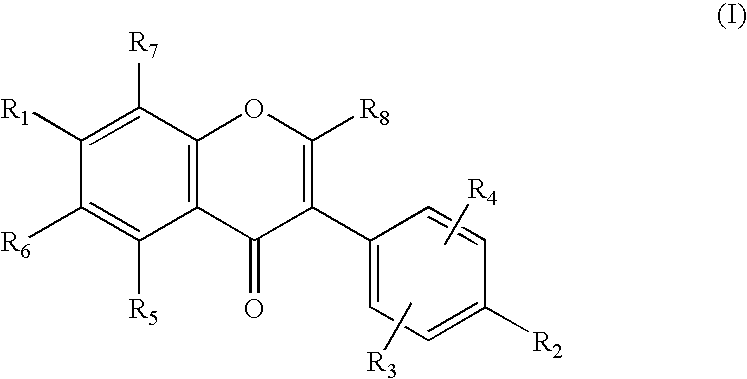
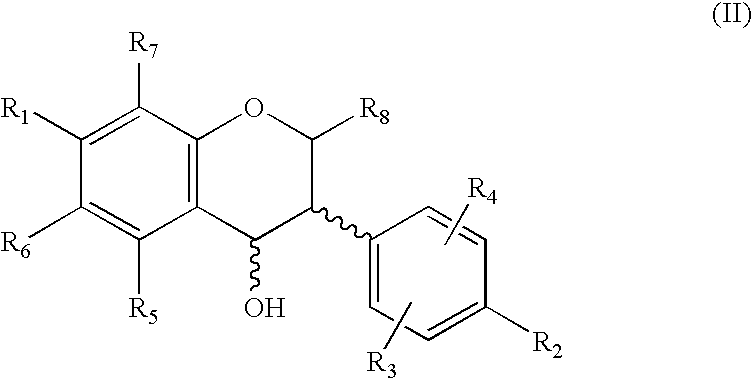
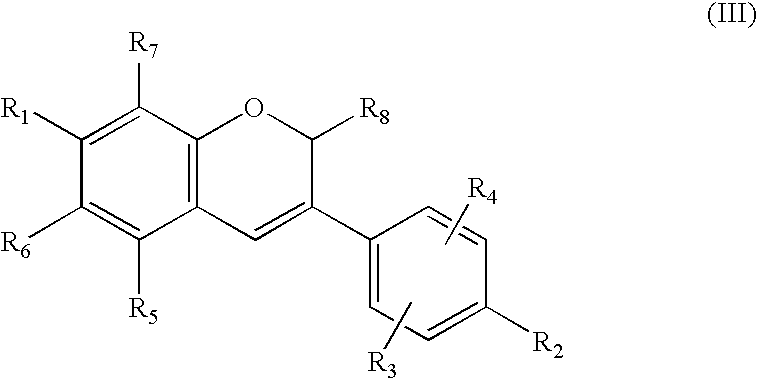
Production of isoflavone derivatives
a technology of isoflavone and derivatives, applied in the field of isoflavone derivative production, can solve the problems of difficult control and lead to mixtures of different products, difficult to separate individual compounds, and general method suitable for large-scale synthesis of these metabolites
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
4′,7 Diacetoxydaidzein
Method A
[0127] A mixture of daidzein (1.0 g, 3.9 mmol), acetic anhydride (5 ml) and pyridine (5 ml) was left in the dark at room temperature for 24 h. The reaction mixture was poured into water (100 ml), stirred for 2 h and then extracted with dichloromethane (3×50 ml). The dichloromethane layer was washed with water, dried over anhydrous sodium sulfate and evaporated. The white residue was crystallised from methanol to yield daidzein diacetate as white prisms (1.1 g, 83%). 1H NMR (CDCl3): δ 2.32 (s, 3H, OCOCH3), 2.36 (s, 3H, OCOCH3), 7.18 (d, 2H, J 9.2 Hz, ArH), 7.19 (d, 1H, J 9.0 Hz, H6), 7.31 (d, 1H, J 20 Hz H8), 7.59 (d, 2H, J 9.2 Hz, ArH), 8.00 (s, 1H, H2), 8.33 (d, 2H, J 8.2 Hz, ArH).
Method B
[0128] A mixture of daidzein (2.0 g, 7.9 mmol), acetic anhydride (10 ml) and pyridine (2 ml) was heated on an oil bath at 105-110 C for 1 h. After cooling the mixture to room temperature, it was stirred for a further 30 min during which time the diacetate crysta...
example 2
7-acetoxy-4′-methoxyisoflavone
[0129] A mixture of 7-hydroxy-4′-methoxyisoflavanone (2.0 g, 7.5 mmol), acetic anhydride (10 ml) and pyridine (2 ml) was heated on an oil bath at 105-110 C for 1 hour. After cooling the mixture to room temperature, it was poured into water (100 ml), stirred for 2 hours and then extracted with dichloromethane (3×50 ml). The dichloromethane layer was washed with water, dried over anhydrous sodium sulfate and evaporated. The white residue was crystallised from methanol to yield 7-acetoxy-4′-methoxyisoflavone as colourless prisms (2.1 g, 91%). 1H NMR (CDCl3): δ 2.36 (s, 3H, OCOCH3), 3.84 (s, 3H, OCH3), 6.98 (d, 2H, J 8.7 Hz, ArH), 7.16 (dd, 1H, J 1.9 Hz 8.6 Hz, H6), 7.30 (d, 1H, J 1.9 Hz H8), 7.50 (d, 2H, J 8.7 Hz, ArH), 8.00 (s, 1H, H2), 8.32 (d, 1H, J 8.6 Hz, HS).
example 3
3′,7-Diacetoxyisoflavone
[0130] 3′,7-Diacetoxydaidzein was prepared from 3′,7-dihydroxyisoflavone (0.98 g, 3.9 mmol), acetic anhydride (6 ml) and pyridine (1.1 ml) as described for 4′,7-diacetoxydaidzein. Yield: (1.0 g, 77%) m.p. 152° C. 1H NMR (CDCl3): δ 2.31 and 2.36 (each s, 3H, OCOCH3), 7.14 (m, 1H, ArH), 7.18 (dd, 1H, J 2.0 Hz 8.6 Hz, H6), 7.31 (d, 1H, J 2.0 Hz H8), 7.37-7.45 (m, 3H, ArH), 8.03 (s, 1H, H2), 8.32 (d, 1H, J 8.6 Hz, H5). Mass spectrum:r m / z 338 (M, 8%); 296 (53); 254 (100); 253 (60).
PUM
| Property | Measurement | Unit |
|---|---|---|
| pressure | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com