Inhibitors of serine proteases, particularly HCV NS3-NS4A protease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiments
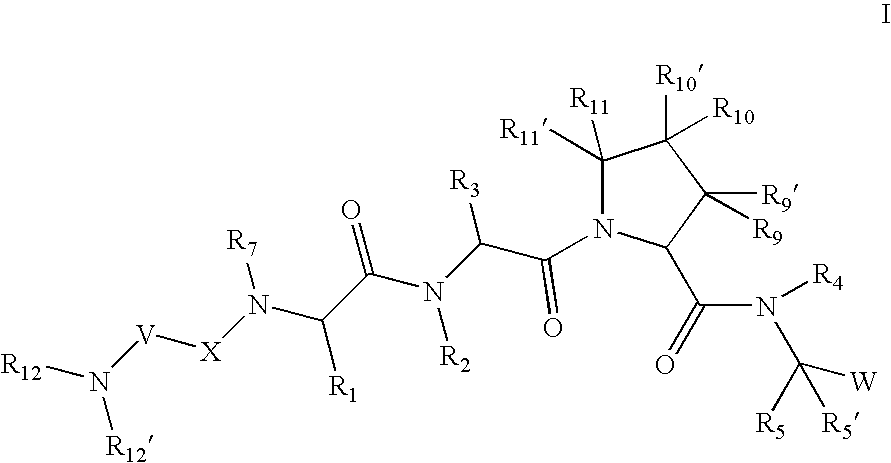
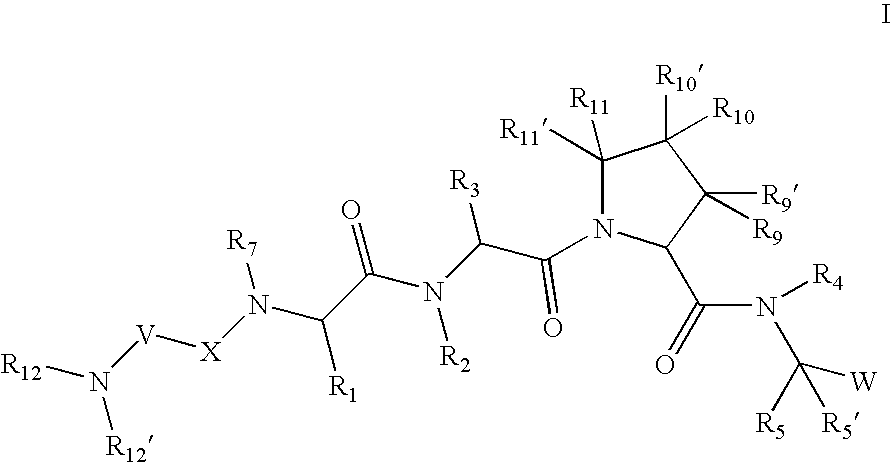
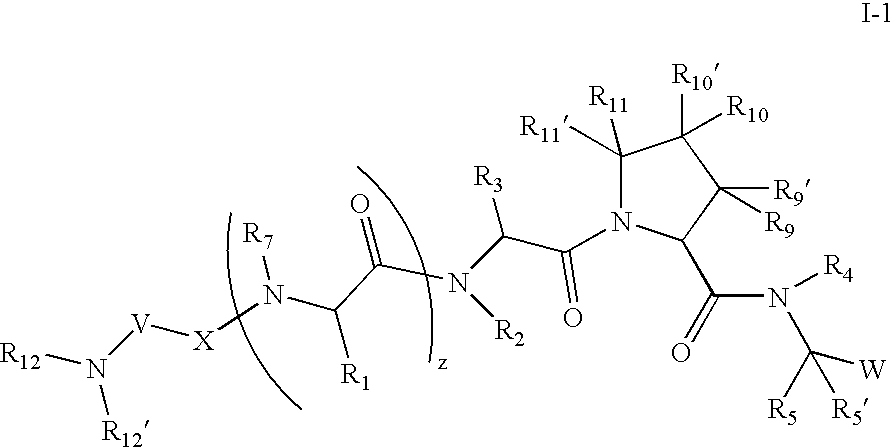
[0266] According to one embodiment for compounds of formula I or formula I-1, the
radical is:
wherein: [0267] R12, R12′, and R13 are as defined in any of the embodiments herein.
[0268] According to another embodiment for compounds of formula I or formula I-1, the
radical is:
wherein: [0269] R12′ is hydrogen; [0270] R12 is: [0271] (C1-C12)-aliphatic-; [0272] (C6-C10)-aryl-, [0273] (C6-C10)-aryl-(C1-C12)aliphatic-, [0274] (C3-C10)-cycloalkyl or -cycloalkenyl-, [0275] [(C3-C10)-cycloalkyl or -cycloalkenyl]-(C1-C12)-aliphatic-, [0276] (C3-C10)-heterocyclyl-, [0277] (C3-C10)-heterocyclyl-(C1-C12)-aliphatic-, [0278] (C5-C10)-heteroaryl-, or [0279] (C5-C10)-heteroaryl-(C1-C12)-aliphatic-; [0280] wherein up to 3 aliphatic carbon atoms in R12 may be replaced by a heteroatom selected from O, N, NH, S, SO, or SO2 in a chemically stable arrangement; [0281] wherein R12 is optionally substituted with up to 3 substituents independently selected from J; and [0282] R13 is as defined in any o...
example 1
N-Phenyl Oxalamic Acid (84)
[0717] Ethyl oxanilate 83 (Aldrich, 1.0 g, 1.0 eq) in 12 mL of THF was treated dropwise with a 1N NaOH solution (5.70 mL, 1.1 eq) resulting in a white precipitate. After stirring for 3 hours at RT, 0.5N HCl and ethyl acetate were added, the organic layer separated, washed with 0.5N HCl and brine and then dried over sodium sulfate, filtered, and concentrated to give 712 mg (68%) of N-phenyl oxalamic acid 84 as a white solid with consistent analytical data. FIA M+H=166.0, M−H=163.9; 1H NMR (DMSO-d6) δ 10.70 (s,1H), 7.75 (m,2H), 7.35 (m,2H), 7.15 (m,1H) ppm.
example 2
N-(Cyclohexyl-{1-[2-(1-cyclopropylaminooxalyl-butylcarbamoyl)-octahydro-indole-1-carbonyl]-2,2-dimethyl-propylcarbamoyl}-N′-phenyl-oxalamide (1)
[0718] Octahydro-indole-1,2-dicarboxylic acid 1-tert-butyl ester 89 (Bachem, 1.6 g, 1.0 eq) in DMF (20 mL) was treated with PyBOP (3.41 g, 1.1 eq) and NMM (1.97 mL, 3.0 eq). To this was added 3-amino-2-hydroxy-hexanoic acid cyclopropylamide 90 (1.22 g, 1.1 eq, prepared according to the procedure of U. Schoellkogf et al., Justus Liebigs Ann. Chem. GE, pp. 183-202 (1976) and J. Semple et al., Org. Letters, 2, pp. 2769-2772 (2000) and NMM (1.97 mL, 3.0 eq) in DMF (3 mL) and the mixture stirred at rt overnight. The mixture was evaporated in vacuo, diluted with ethyl acetate, the organic phase washed with 0.5N HCl, sodium bicarbonate and brine, then dried over sodium sulfate, filtered and concentrated in vacuo to give 2-[1-cyclopropylcarbamoyl-hydroxy-methyl)-butylcarbamoyl]-octahydro-indole-1-carboxylic acid tert-butyl ester 91 which was used w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Energy | aaaaa | aaaaa |
| Composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com