Intradermal needle
a needle and intradermal technology, applied in the field of needle assembly, can solve the problems of insufficient immune response, inability to perform the standard intradermal injection procedure, and difficulty in the technique used to perform the standard intradermal injection, and achieve the effect of effective and reliable delivery of such substances intradermally
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
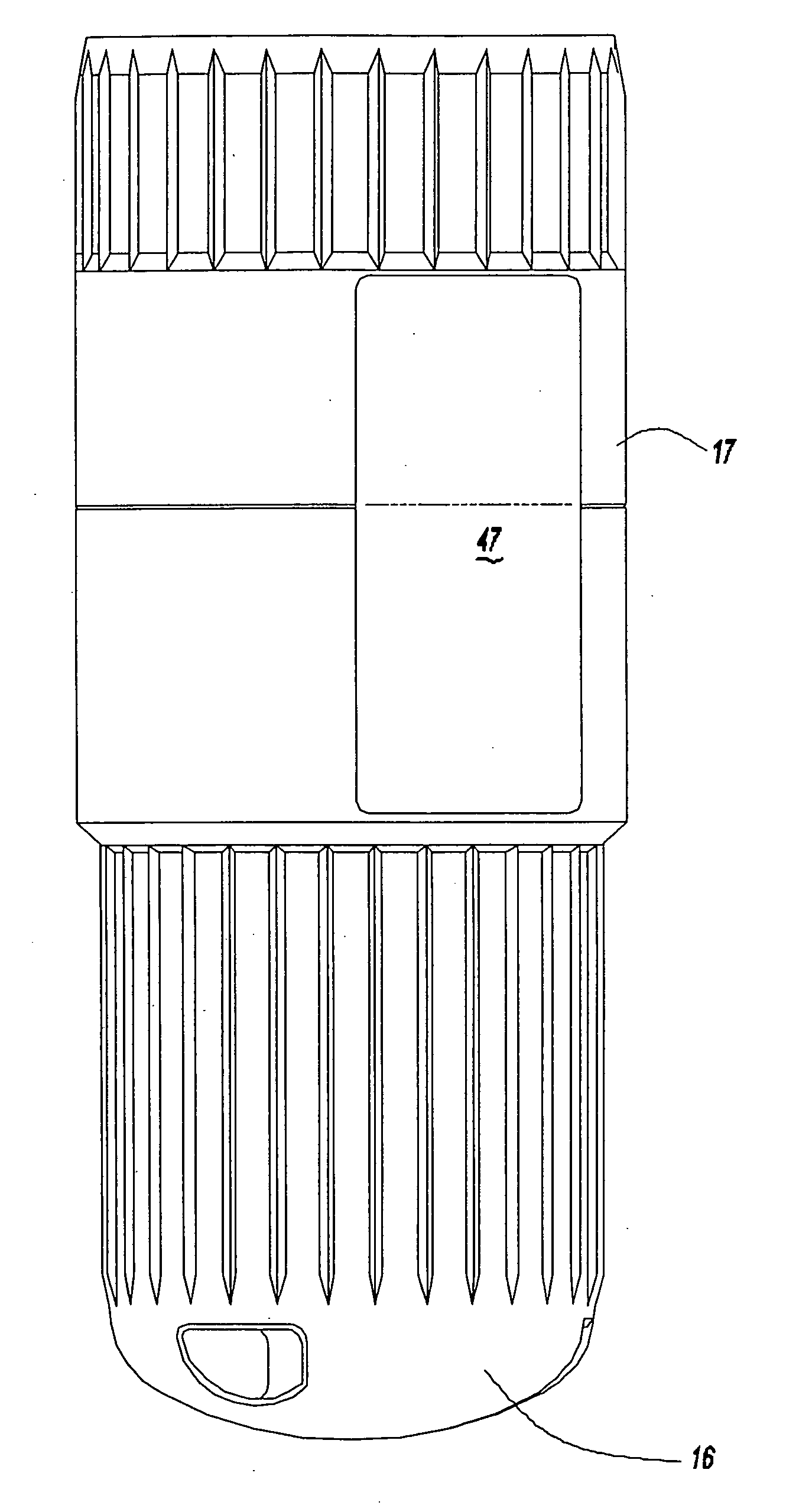
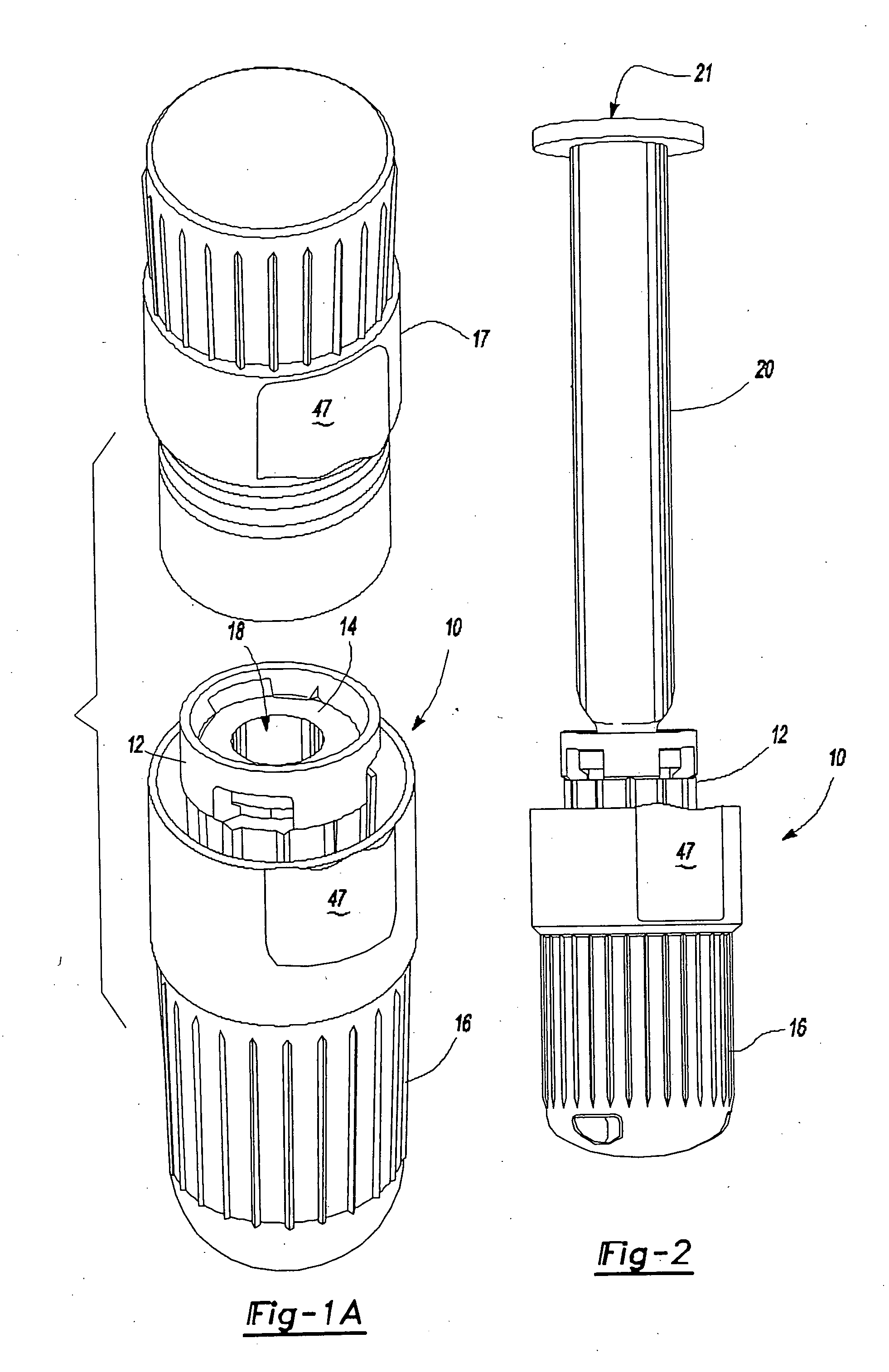
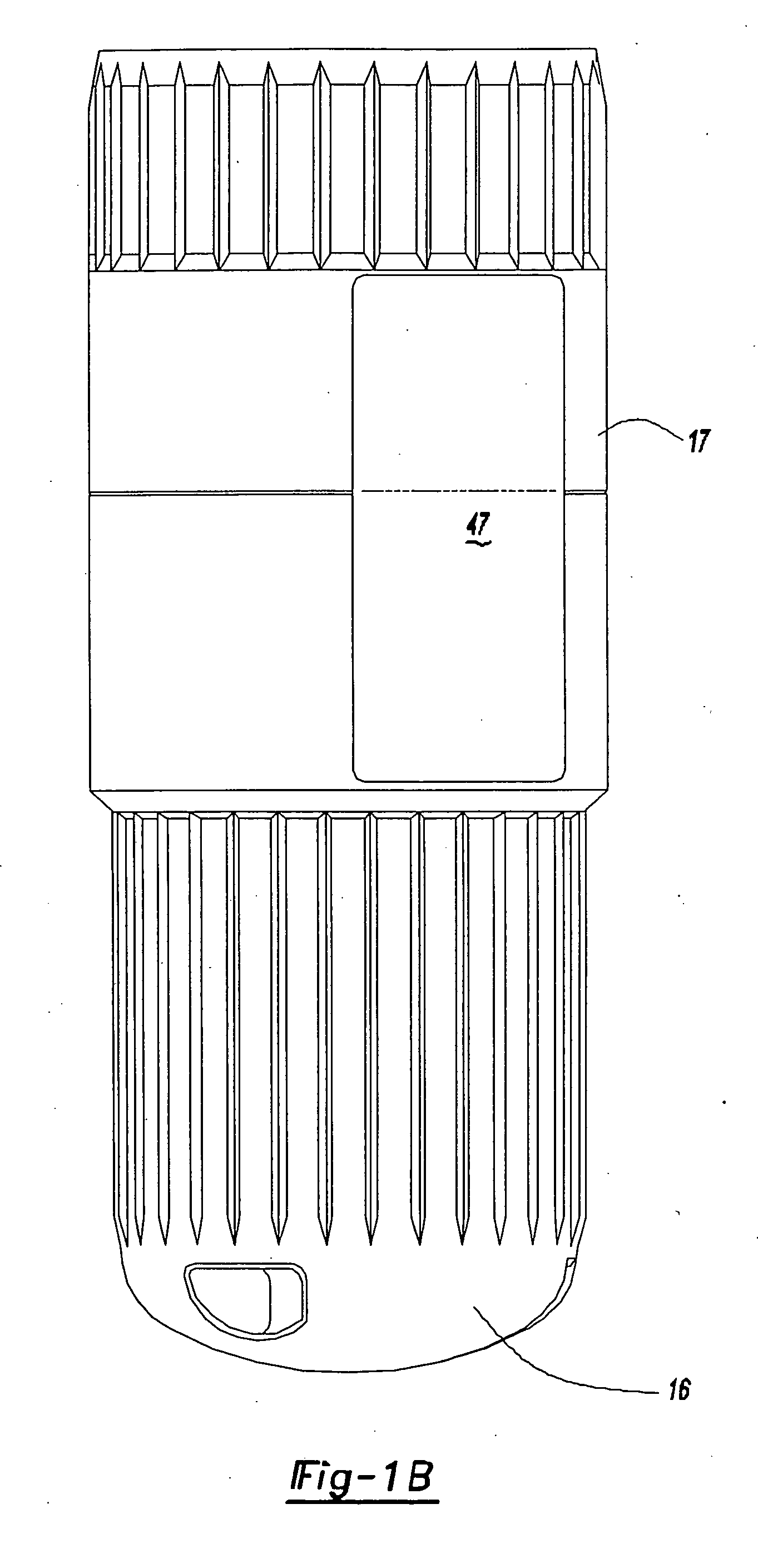
[0038] Referring to FIGS. 1A and 1B, an intradermal needle assembly is generally shown at 10. The assembly includes a limiter portion 12 and a hub portion 14 disposed inside the limiter portion 12. A forward cap 16 is disposed upon the end of the hub portion 14, and a rearward cap 17 is removably affixed to the forward cap 16, the purpose of which will be explained further below. The hub portion 14 includes a throat 18 adapted to receive a prefillable container 20, as shown in FIG. 2.
[0039] The prefillable container 20 includes a reservoir 21 adapted to store substances intended for intradermal delivery into the skin of an animal. The substances comprise drugs or vaccines known to be absorbed into or react with the immune response system of the body significantly better in the dermis layer of the skin of the animal as opposed to in the subcutaneous or intramuscular region of the animal. Specifically, hepatitis B vaccines, it has been determined, are significantly more imunogenic wh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| distance | aaaaa | aaaaa |
| distance | aaaaa | aaaaa |
| distance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com