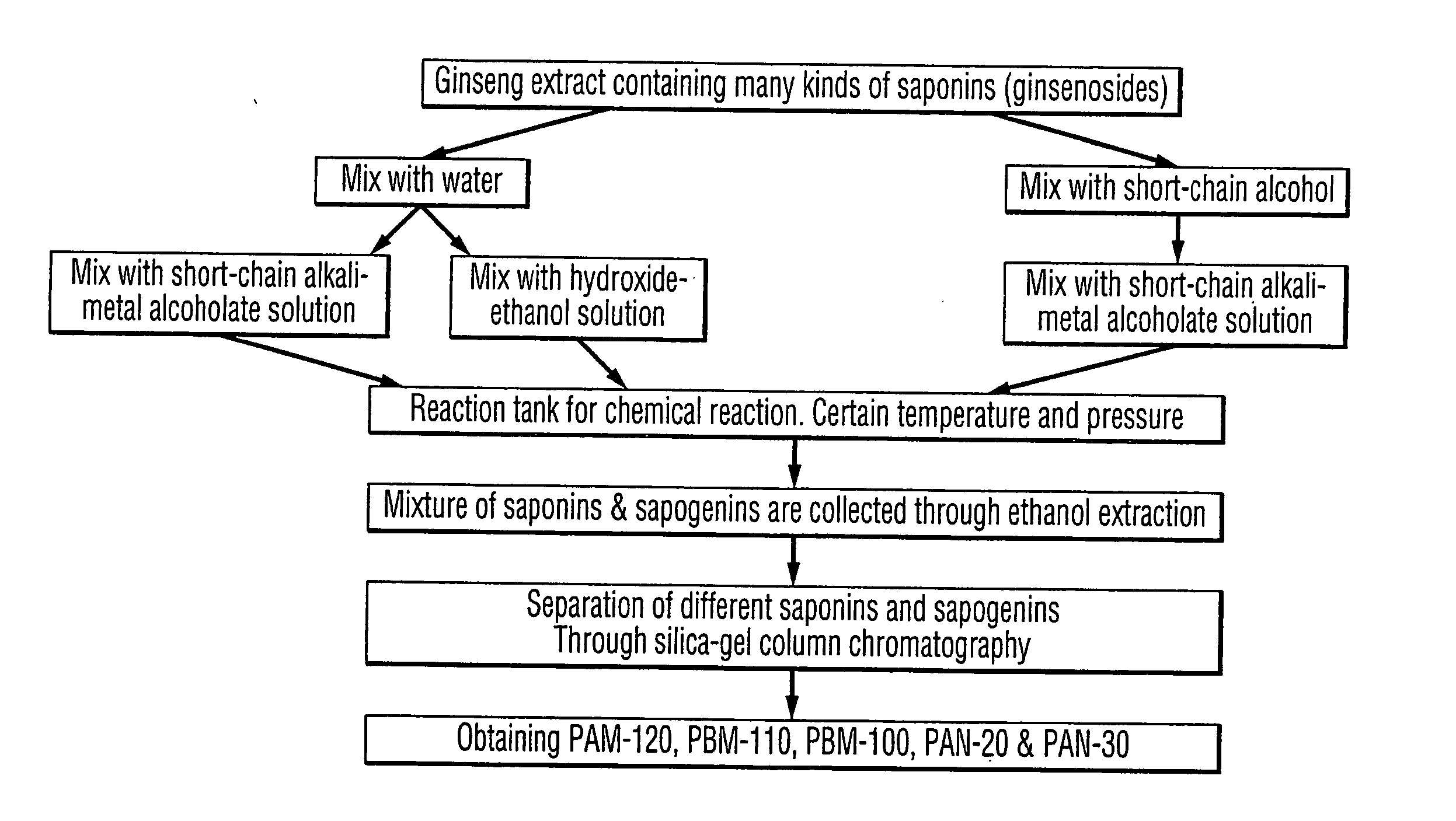
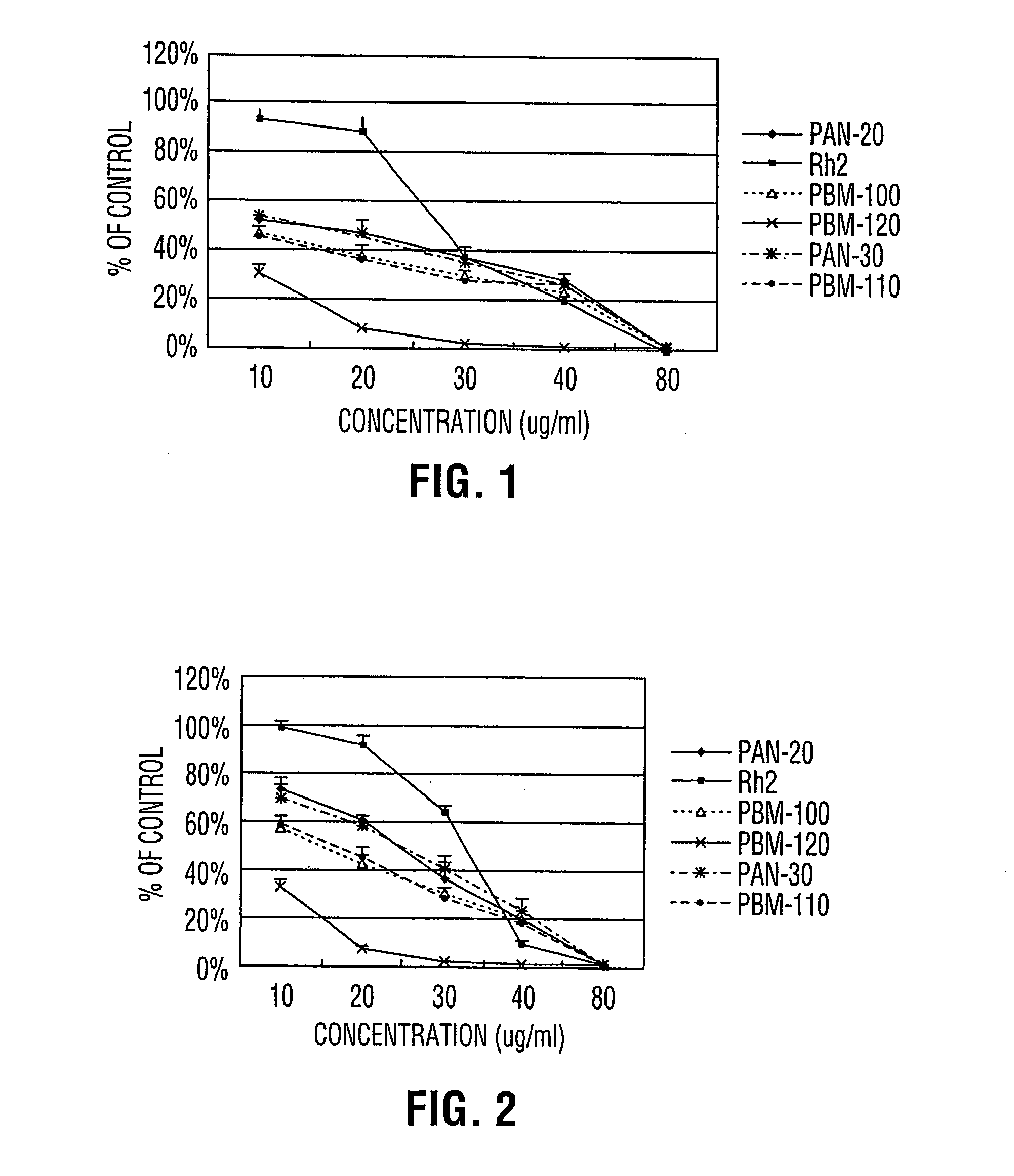
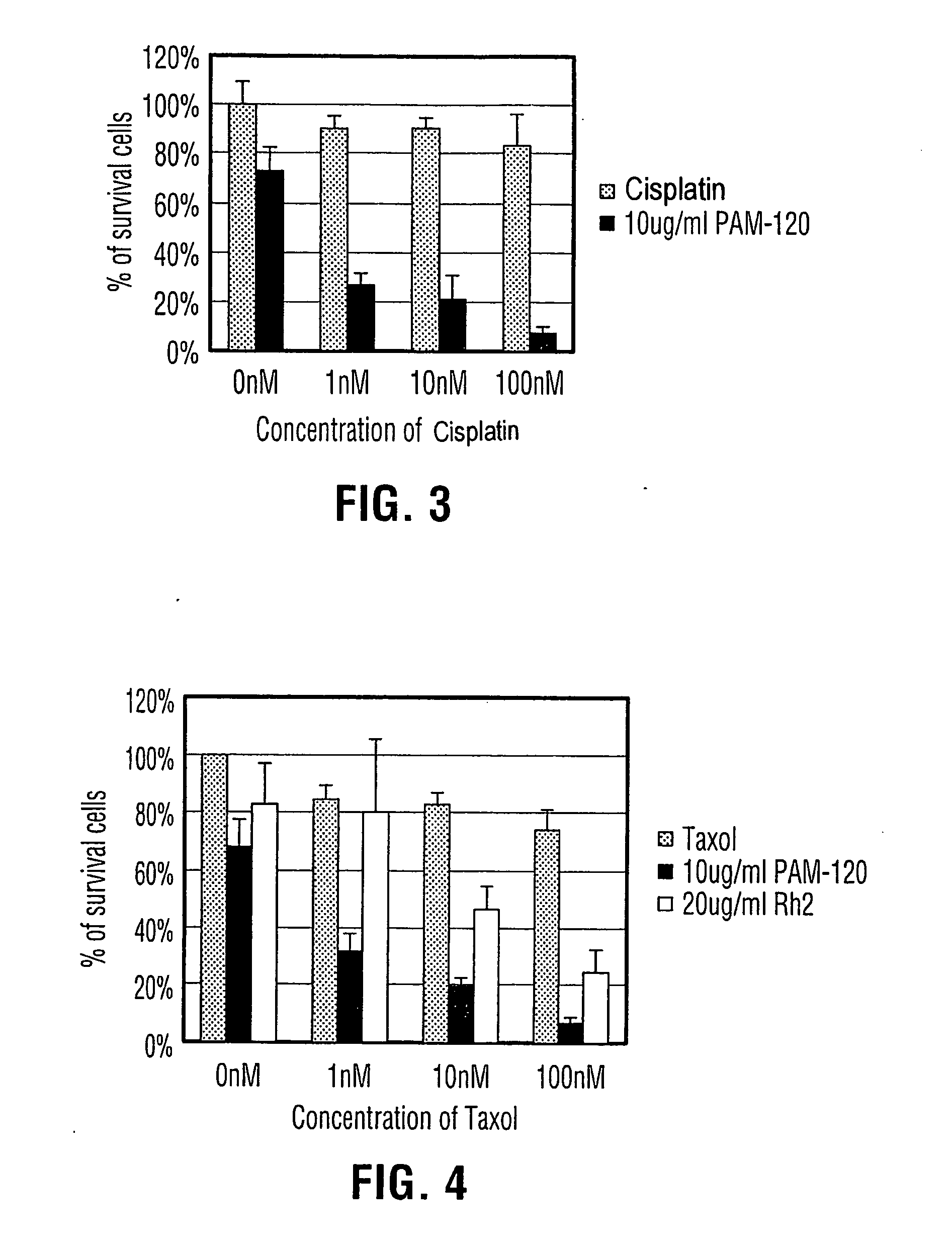
Process for producing dammarane sapogenins and ginsenosides
a technology of dammarane sapogenin and ginsenoside, which is applied in the field of dammarane sapogenin, can solve the problems of difficult if not impossible separation of 20(r) and 20(s) epimers, and achieve the effect of surprising anti-cancer effect, unexpected and superior activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation Process of Producing PAM-120, PBM-100, and PAN-20
[0111] [1] Ginseng crude extract 10 g was dissolved in 40 mL of 95% ethanol [0112] [2] Add 40 mL of 5 N NaOH [0113] [3] Pour into the reaction tank, and set temperature to 240° C., and pressure to 3.5 Mpa, for 1.5 hours [0114] [4] Reduce temperature to room temperature, and take the products out the tank [0115] [5] Add HCl to neutralize pH to about 7, and expend the volume to 800 mL using water [0116] [6] Extract 3 times with acetic ester, 100 mL each time [0117] [7] Combine all the extracts, and reduce the pressure to dry. Thus, obtain 3.8 g of dried extracts [0118] [8] Grind and dissolved the extract in 20 mL of methanol, and mix the methanol solution with silica gel [0119] [9] Dry up the mixture, and then grind to fine powder [0120] [10] Load the Silica gel column [0121] [11] Wash the column with 60 mL of ether:petroleum benzin (1:3), and thus, 250 mg of PAM-120, and 45 mg of PBM-100 were obtained [0122] [12] Wash the ...
example 2
Another Example of Preparation Process Producing PAM-120, PBM-100, and PAN-20
[0123] [1] 10 g of Ginseng crude extract was added into reaction tank [0124] [2] Add to the reaction tank 100 mL of 5 N NaOH [0125] [3] Set temperature to 270° C. and pressure to 4.5 Mpa for 1 hour [0126] [4] Reduce temperature to room temperature, then take out the products [0127] [5] Neutralize the pH to 7 using HCl [0128] [6] Filter and keep the solids [0129] [7] Dissolve the solids in 10 mL of 95% Ethanol [0130] [8] Add water to make ethanol content less than 5% [0131] [9] Sit still overnight [0132] [10] Filter and keep the solids [0133] [11] Dry the solids [0134] [12] Dissolved the solids in 10 mL of methanol [0135] [13] Filter and keep the solution [0136] [14] Dry the solution to obtain 3.6 g of products [0137] [15] Mix the products with 11 g of silica gel [0138] [16] Grind and then load the silica gel column [0139] [17] Wash the column with 100 mL of ether:petroleum benzin (1:3), and thus, 60 mg of ...
example 3
Preparation Process of Producing 20(S) / 20(R) Protopanaxadiol, 20(S) / 20(R) Protopanaxatriol and 20(S) / 20(R) Ginsenoside Rh2
[0141] [1] Ginseng crude extract 10 g was dissolved in 40 mL 95% ethanol [0142] [2] Add 40 mL 5 N NaOH [0143] [3] Pour into the reaction tank, and set temperature to 240° C., and pressure to 3.5 Mpa, for 1.5 hours [0144] [4] Reduce temperature to room temperature, and take the products out [0145] the tank [0146] [5] Add HCl to neutralize pH to about 7, and expand the volume to 800 mL using water [0147] [6] Extract 3 times with Ethyl acetic(EtoAc), 100 mL each time [0148] [7] Combine all the extracts, and reduce the pressure to dry. Thus, obtain 3.8 g of dried extracts [0149] [8] Grind and dissolved the extract in 20 mL of methanol, and mix the methanol solution with silica gel [0150] [9] Dry up the mixture, and then grind to fine powder [0151] [10] The powder was subjected to Silica gel column chromatography [0152] [11] Eluted with 60 mL of petroleum ether:EtOAc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com