Autoantigenic fragments, methods and assays
a technology of autoantigen and autoantigen, applied in the direction of genetic material ingredients, instruments, antibody medical ingredients, etc., can solve the problems of non-caspase substrates for granzyme and granule-induced cell death that have not been defined, and achieve the effect of lessening the impact of lessening at least one symptom of the diseas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
General Materials and Methods
[0062] Materials. Purified DNA-dependent protein kinase (DNA-PK) and SP1 were purchased from Promega (Madison, Wis.). ATP was purchased from Fluka (Ronkonkoma, N.Y.), and 32P-ATP was from Du Pont / NEN (Wilmington, Del.). Ac-DEVD-CHO and Ac-YVAD-CHO were manufactured by Merck (Rahway, N.J.). Caspase-3 was purified as described (Nicholson et al., 1995). Patient sera were used to immunoblot the nuclear mitotic apparatus protein (NuMA), poly(ADP-ribose) polymerase (PARP) and DNA-PKCS (Casciola-Rosen et al., 1995; Greidinger et al., 1996). Monoclonal antibodies can be made by methods known in the art. Two different monoclonal antibodies, designated 18-2 and 25-4 (kind gifts from Dr. Tim Carter, St. Johns University, Jamaica, N.Y.) were also used to detect DNA-PKCS by immunoblotting (see Table II for a summary of the antibodies used to detect DNA-PKCS and its cleaved fragments). Rabbit polyclonal antibodies to caspases were raised against the large subunits o...
example 2
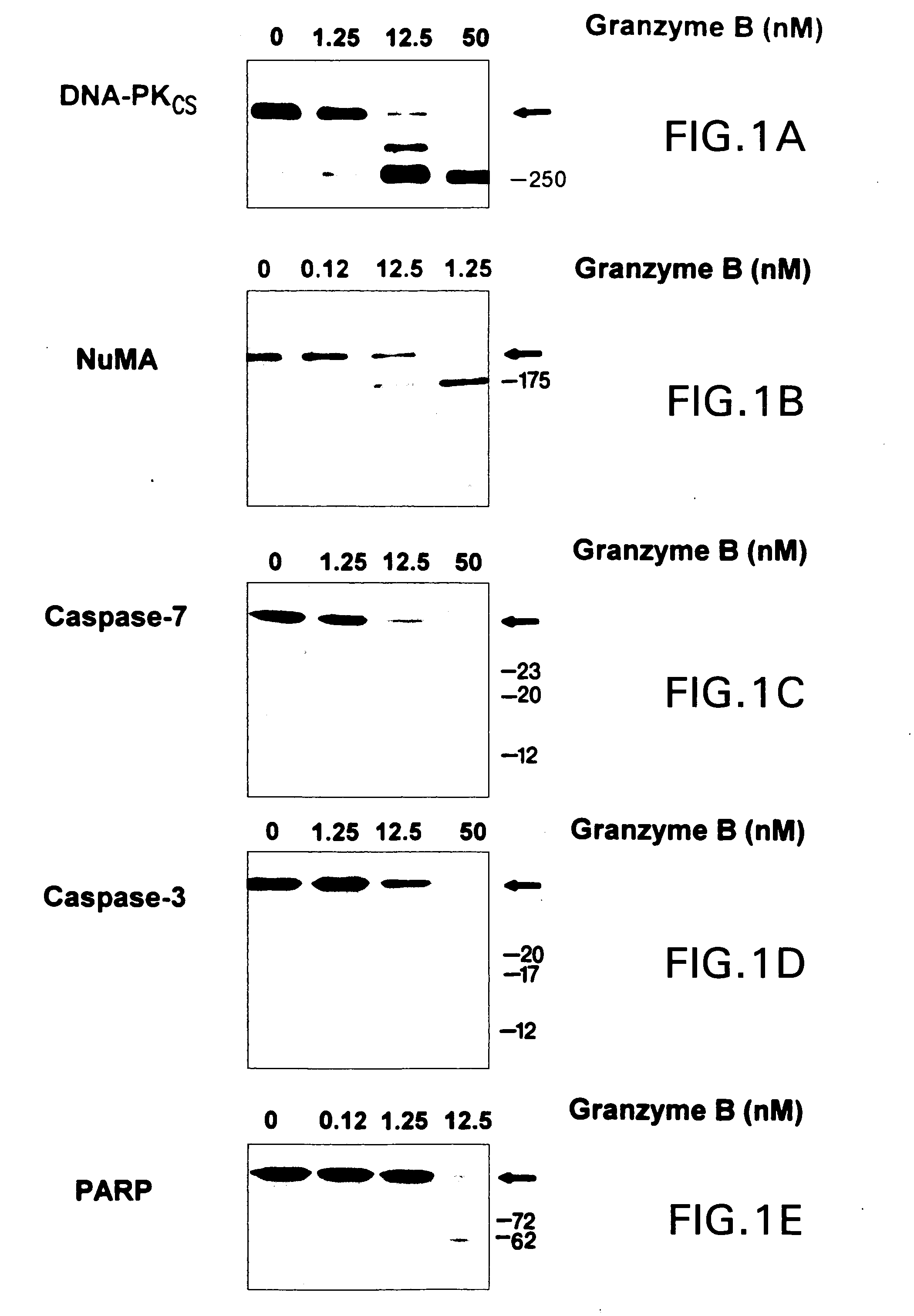
DNA-PKCS and NuMA are Very Efficient Substrates for Purified Granzyme B.
[0071] Granzyme B has previously been reported to cleave the precursors of several caspases (including caspases 3, 7 and 10), resulting in activation of their proteolytic activity. The catalytic efficiency of cleavage of these substrates by granzyme B serves as a useful standard against which granzyme B-mediated cleavages of other substrates can be compared. Purified [35S]methionine-labeled precursors of caspase-3 and caspase-7, or THP.1 cytosols (containing these precursor proteases) were incubated in vitro with increasing concentrations of purified granzyme B. The dose-response data obtained (FIG. 1) was used to calculate catalytic constant (kcat / Km) values of 1.8±0.6×105M−1s−1 (radiolabeled substrate) and 1.9±0.1×105 M−1s−1 (immunoblotting) for caspase-7, and 3.6±1.0×104 M−1s−1 (radiolabeled substrate) and 2.3±0.4×104M−1s−1 (immunoblotting) for caspase-3 (Table I). Thus, granzyme B cleaves caspase-7 approxi...
example 3
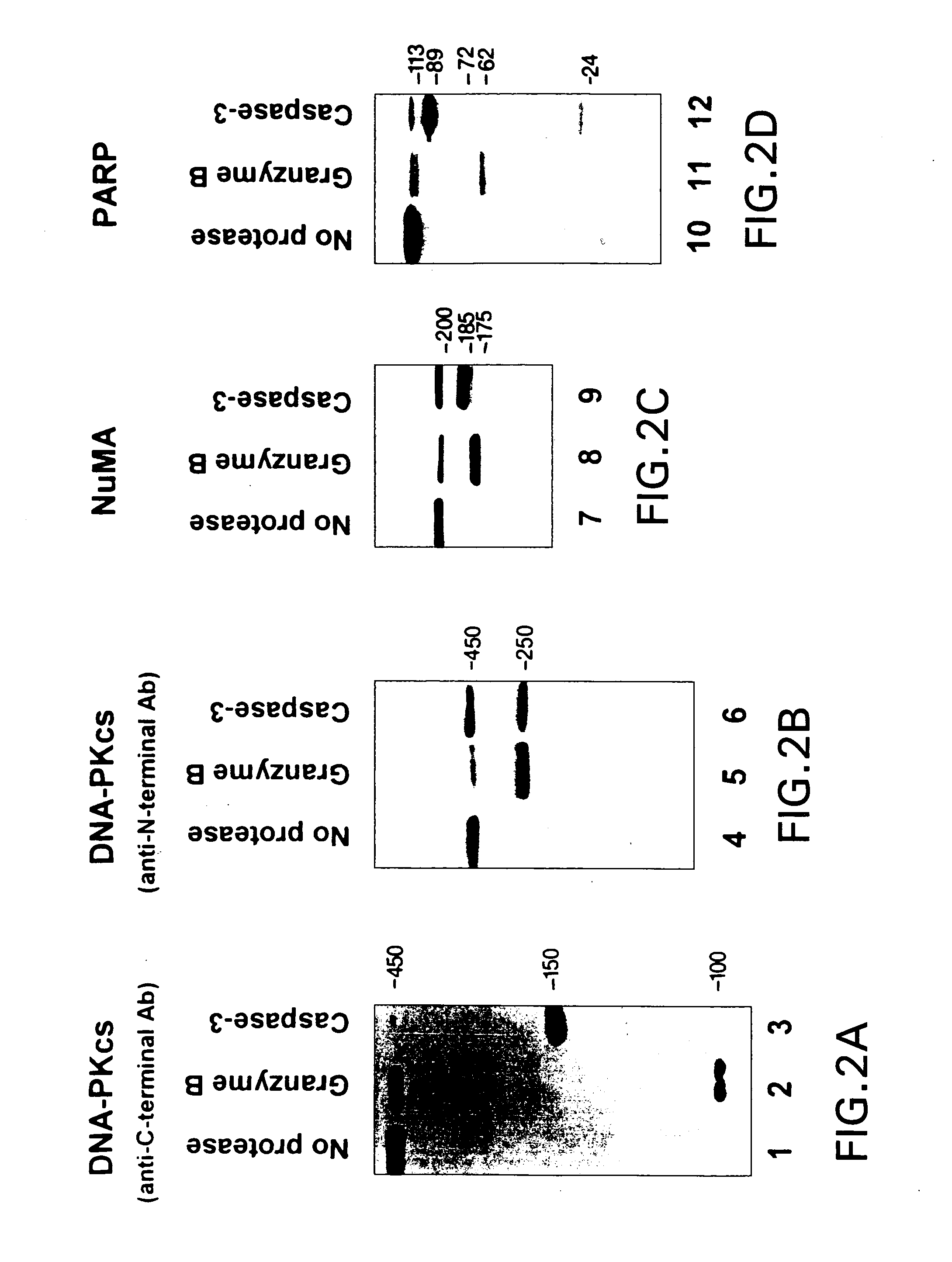
Different Substrate Fragments are Detected After Cleaving Autoantigens in Vitro with Granzyme B or Caspase-3.
[0074] To directly compare the fragments generated by granzyme B and caspase-3, purified DNA-PKCS, in vitro translated [35S]methionine-labeled PARP, and endogenous substrates (NuMA and DNA-PKCS in HeLa cell lysates) were incubated with protease and electrophoresed in adjacent lanes. When granzyme B was used to cleave DNA-PKCS, fragments of 100 kDa and 250 kDa were generated, (detected by immunoblotting using antibodies recognizing the C-terminus or N-terminus of DNA-PKCS, respectively) (FIG. 2, lanes 2 & 5; and Table II). In contrast, caspase-3 cleavage yielded a 150 kDa C-terminal fragment (FIG. 2, lane 3) and a 250 kDa N-terminal fragment (FIG. 2, lane 6).
[0075] Granzyme B-mediated cleavage of NuMA generated a novel fragment migrating at 175 kDa on SDS-PAGE, which was distinct from the 185 kDa fragment detected after cleavage with caspase-3 (FIG. 2, lanes 7-9). Similarly...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com