Of particular interest to us is the possibility of screening for very short-lived secreted products on the basis of changes in migration patterns or morphology or phenotypic marker expression of cells in the immediate vicinity of transfected or otherwise engineered “secretor cells.” Such short-lived products may exist and have important roles in physiological processes, but being short-lived, they would not be readily detectible due to their
instability under normal circumstances.
Although the non-adherence of blood cells
in vivo is implicit, the non-adherent nature
in vitro of many types of hematopoietic cells is not so readily accepted.
Most theories of
cell migration and motility require the involvement of molecular attachment of
cell adhesion molecules to the surface, for example through
integrin-mediated binding to
fibronectin (DiMilla et al., 1993; Lauffenburger, 1996; Maheshwari et al., 1999), and there is as yet no satisfactory theory for how non-adherent cells migrate.
However, it has also become clear that major components of the migratory “behavior” of these cells are non-biological influences of gravity and micro-turbulence, probably due to thermal
convection.
Given such problems, and despite the appeal of video time-lapse imaging for gathering otherwise unobtainable information relating to detailed characteristics of
cell migration, there are as yet no validated methods described in the literature for 2 dimensional migration analysis of non-adherent cells.
This method is based upon the use of 3D collagen gels and does not allow for analysis of motion that is achieved apart from surface adhesion.
However, the incorporation of these cells into a 3D collagen environment leads to the onset of spontaneous migration; this results in the rapid and persistent
tyrosine phosphorylation of FAK, implicating FAK in
T cell migration.” (Entschladen et al., 1997).
It is suggested that the failure to adequately control ambient motion is the reason why a validated, reproducible method has not yet been put forth for analysis of motion in a 2D environment with non-adherent cells.
Likewise, no method has been presented for analysis of 3D motion in the absence of a
solid matrix (e.g. collagen) upon which cells can attach.
In summary, there are apparently no validated methods in the literature for analysis of migration of T cells or other non-adherent cells on a 2D surface or for analysis of migration in 3 dimensions when there is no
solid matrix on which the cells can attach.
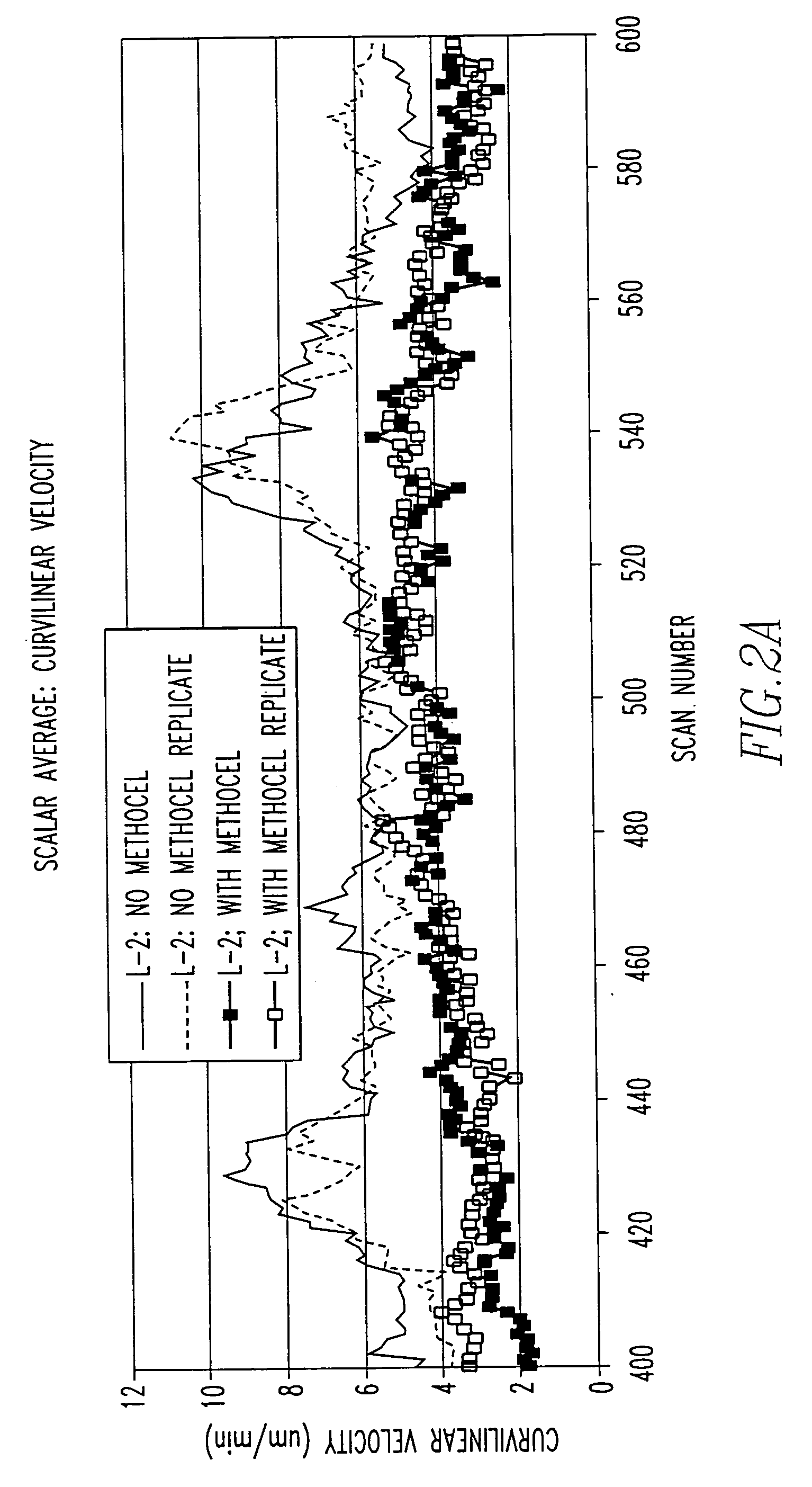
When
methyl cellulose is used, this dissolved compound is not considered to provide attachment surfaces for the cells to adhere to.
In medium alone, frequently it is difficult to distinguish biological from thermal and other types of ambient motion, and in many cases the ambient motion is not of uniform direction across the viewfield, nor is it constant over time.
Therefore, methods to mathematically “correct” for ambient motion will have
noise (uncertainty in precision) associated with them, and in many cases this
noise will be greater than the magnitude of
biological motion.
While an abundance of valuable data has been obtained using the Boyden chamber, this method “also had the drawback that it was now possible to spend a research career studying leukocyte
chemotaxis without ever looking at a moving cell or, indeed, knowing its front from its back ¼”, according to Wilkinson (Wilkinson, 1990).
However, the method is subject to variability depending upon how the holes are bored, perhaps due to lifting of the
agarose from the glass surface allowing cells to migrate along with channeling fluid rather than through biological motility.
Although all of these methods provide a
quantitative measure of migratory activity, their shortcomings leave many aspects of the migratory behavior hidden from the investigator.
 Login to View More
Login to View More