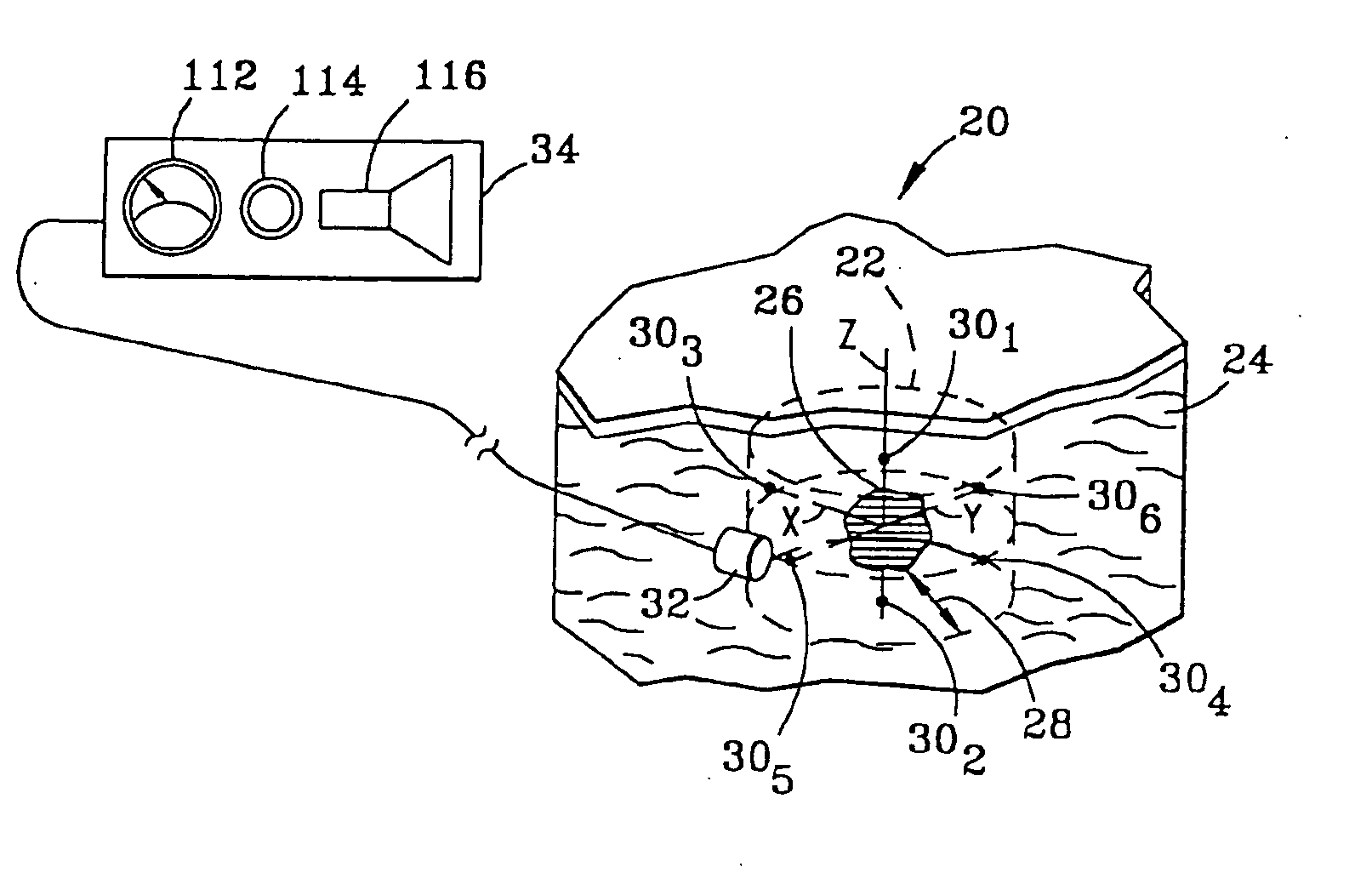
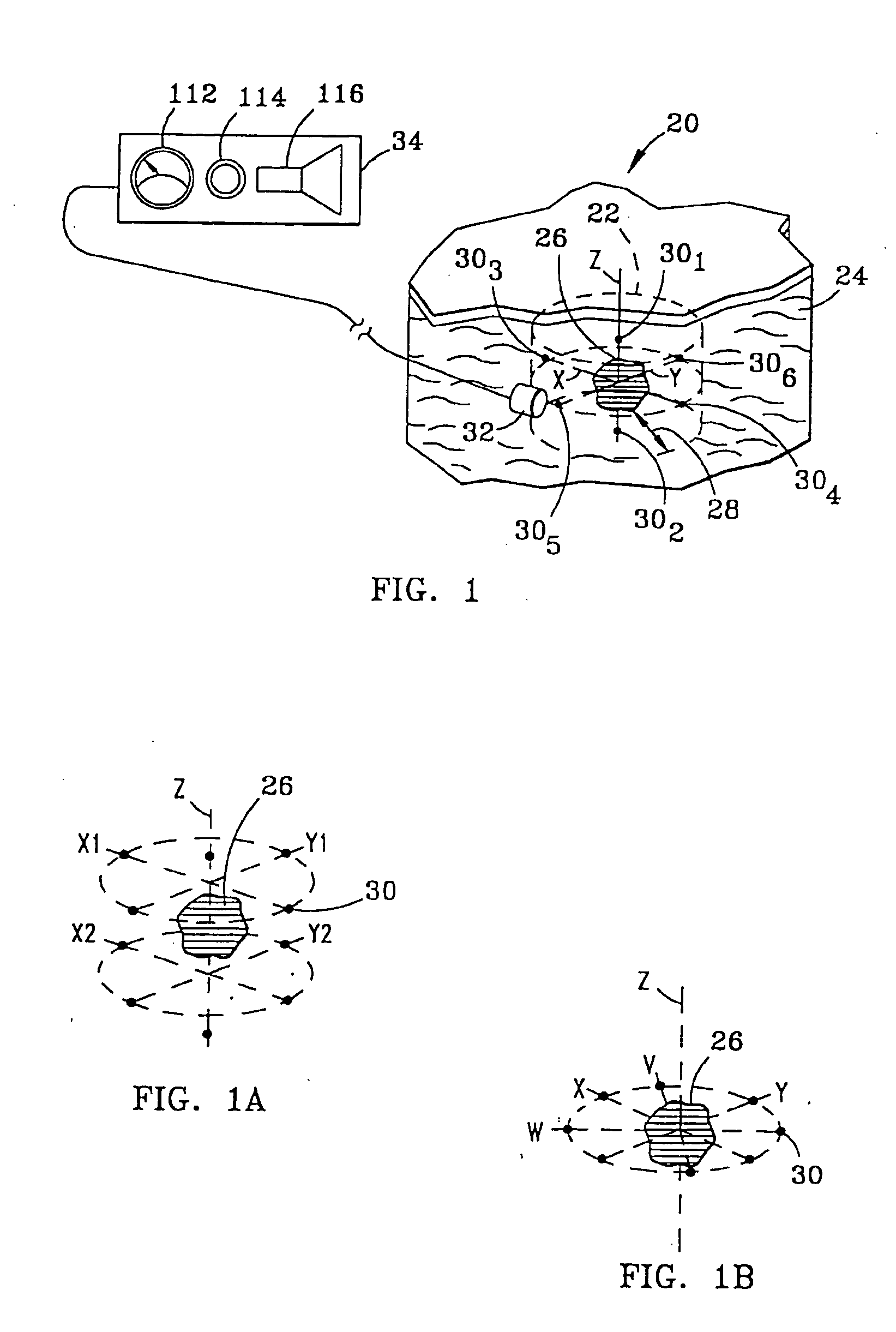
System and method for bracketing and removing tissue
a tissue bracketing and tissue technology, applied in the field of tissue bracketing and tissue removal, can solve the problems of difficult for surgeons to detect the exact depth of the lesion, the technique is not optimal, and the margins of tissue to be removed are difficult to properly define, so as to reduce the mobility of tissu
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
first embodiment
[0048] In addition, markers 30 each have a detection characteristic to enable detection by probe 32 and detector 34. The detection characteristics of the various embodiments of markers 30 can be characterized as active or passive. In the active category, the detection characteristic of marker 30, illustrated in FIG. 2a as marker 30a, is gamma rays 40. In this regard, marker 30a may include materials such as technetium 99, cobalt isotopes or iodine isotopes. Such materials may be obtained from DuPont of Billerica, Mass. Preferably, each marker 30a generates gamma rays 40 having a field strength in the range of 1-100 microCurries.
[0049] Also in the active category, in a second embodiment of marker 30, illustrated in FIG. 2b as marker 30b, the detection characteristic is magnetic field 42. Markers 30b of the second embodiment thus contain ferromagnetic materials in which a magnetic field can be induced, or alternatively are permanently magnetized and so have an associated permanent mag...
third embodiment
[0050] Referring to FIG. 2c, in a third embodiment, again in the active category, marker 30c emits radio frequency (“RF”) signal 44 in response to a triggering signal 46. Various energy sources may be used for triggering signal 46, including a magnetic field, ultrasound or radio frequency energy. In this latter case, marker 30c is preferably designed to receive triggering signal 46 which has a first RF wavelength, and in response thereto, emit signal 44 of a second RF wavelength. In the simplest case, no data, other than the specific radio frequency itself, is carried in signal 44. Alternatively, markers 30c may all transmit signal 44 at a single frequency, with data uniquely identifying each marker being carried in signal 44 emitted by each marker.
[0051] A suitable marker 30c is illustrated in FIG. 3a. This marker 30c includes a transmit / receive antenna 52 for receiving an RF signal at a first frequency and transmitting an RF signal at a second frequency. Also included is a power d...
fourth embodiment
[0053] In a fourth embodiment, again in the active category, marker 30d, illustrated in FIG. 2d, continuously emits signal 44 at specific frequencies in the radio frequency spectrum. The marker 30c illustrated in FIG. 3a and described above can be satisfactorily employed as marker 30d by adding a battery (not shown) in place of power detector portion of circuit 54 of marker 30c. RF exciter 60 is not required in connection with marker 30d, insofar as the battery generates the energy used by the marker in producing RF signal 44.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com