Medical device systems implemented network scheme for remote patient management
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
first embodiment
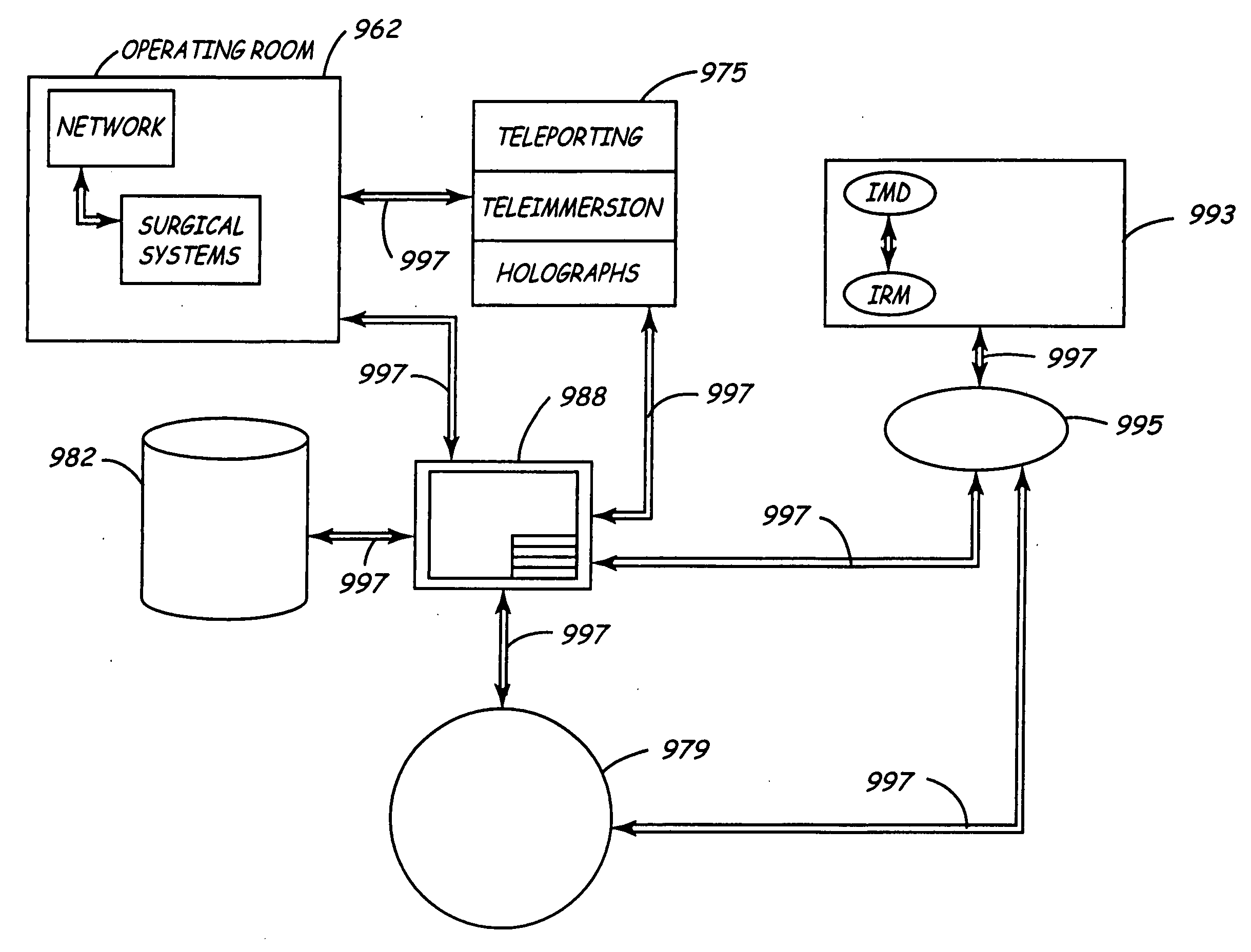
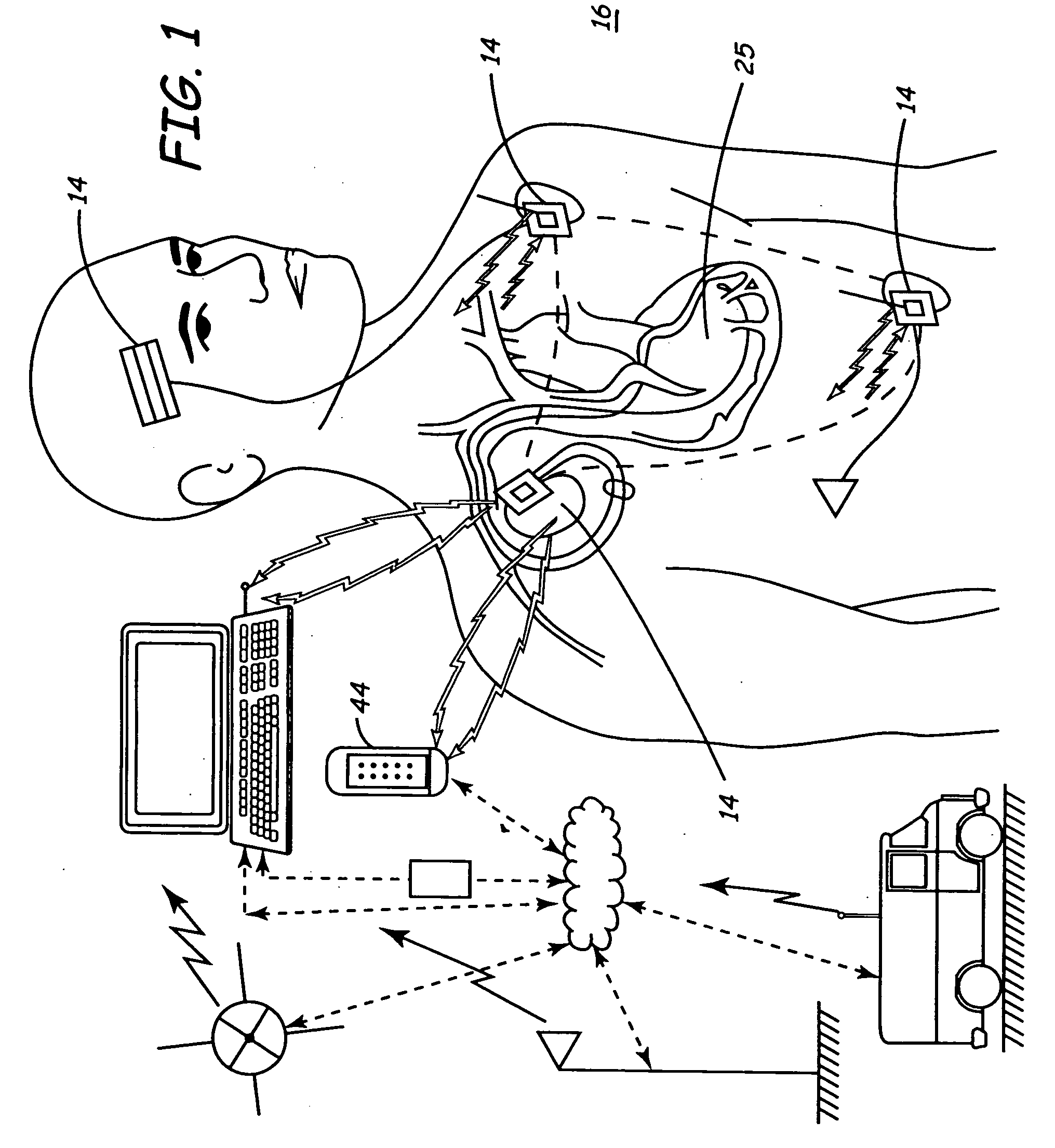
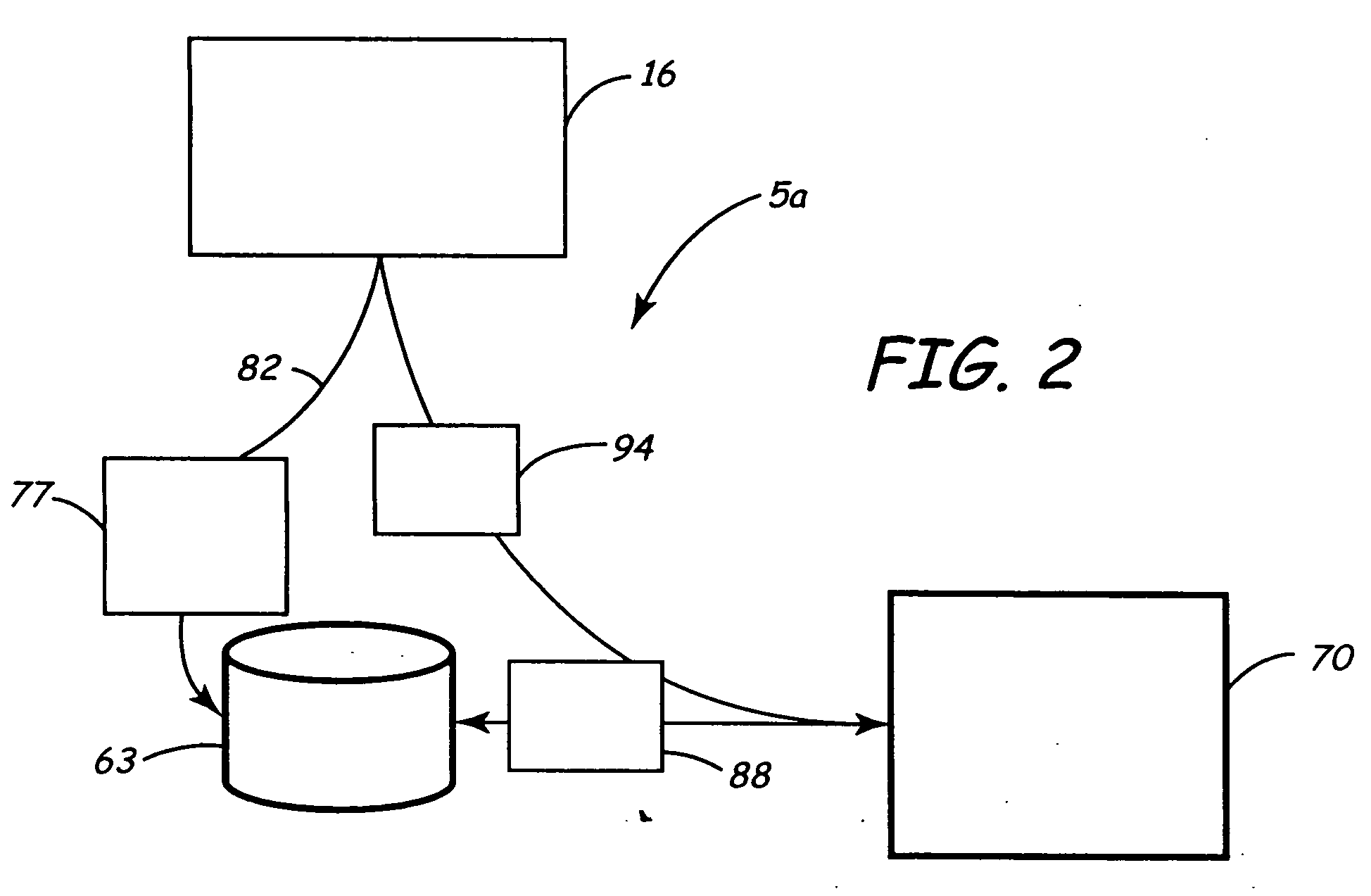
[0051] A first embodiment shows the data 702 flowing to a research and development department 719. It is anticipated that having access to large and highly relevant databases of patient physiology and device performance, will be a valuable source of information in the design of new devices.
second embodiment
[0052] A second embodiment shows the data 702 flowing to the product planning department 729. Knowing how devices are programmed, device longevity, and potential failure modes will be usefully in planning new features and new devices. This is information which is very valuable and available from data 702.
third embodiment
[0053] A third embodiment shows the data 702 flowing to the department responsible for post market surveillance 739. Many governmental entities, e.g., countries, require manufacturers to perform post-market surveillance on their medical devices to understand device performance and failure modes and mechanisms. Having this data available through the server 405 will allow more accurate, timely, and complete information capture; all of which is highly valuable. Indeed, for this and other uses, the particularly high volume of highly relevant data in a full cycle system distinguishes this invention from others.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com