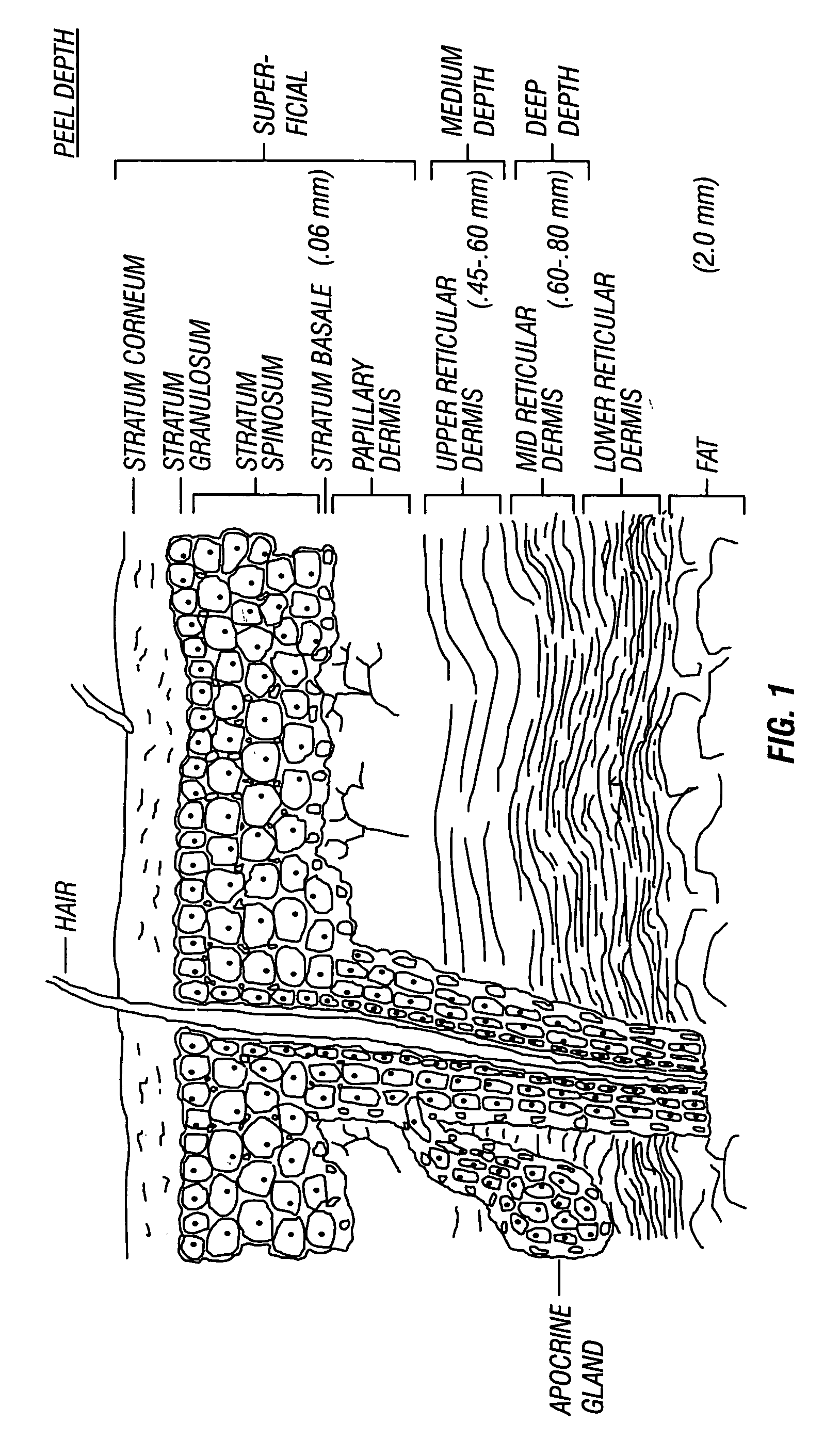
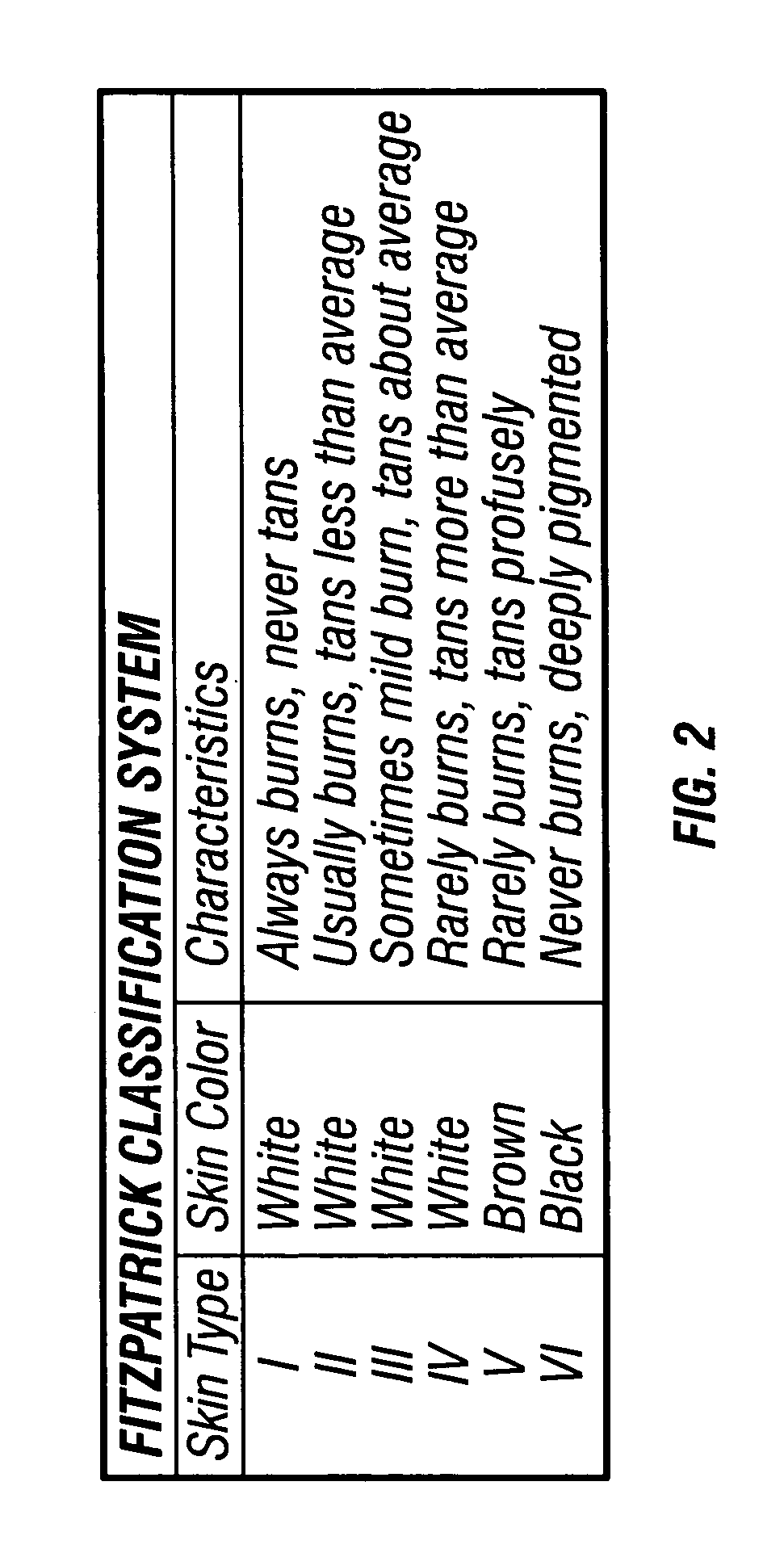
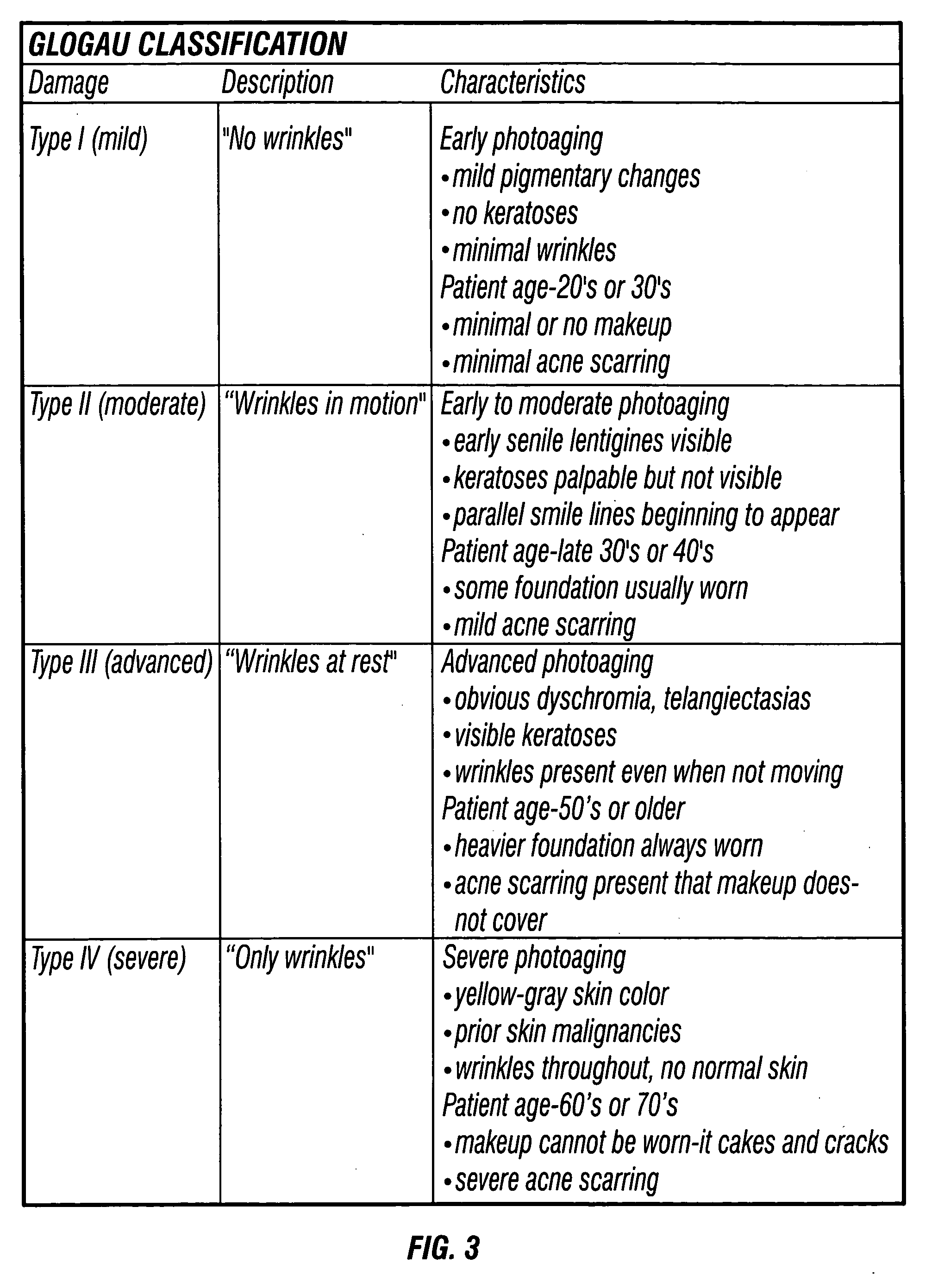
Hydroxy-kojic acid skin peel
a technology of hydroxykojic acid and skin peel, which is applied in the direction of plant growth regulators, biocide, animal husbandry, etc., can solve the problems of direct toxic phenol to the myocardium, hepatotoxic and nephrotoxic, and significant complications of the aforementioned deep phenol peel, so as to achieve safe and efficient peeling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0047] The following is a general procedure or method for application of the selected superficial or intermediate peels of the present invention which may be applied on a periodic basis:
[0048] a. thorough cleansing of facial skin to be peeled using an appropriate degreaser such as alcohol, acetone, freon or chlorhexidine. Since this is meant to be a superficial type of peel, there is no need to do an aggressive scrub to clean the skin. The goal is to degrease the skin, not to strip off any remaining stratum corneum.
[0049] b. apply light coating of the selected peel (as formulated in the following examples) using a synthetic fiber fan brush or equivalent, starting at the chin and working upwards with particularly thorough coverage of hyperpigmented areas;
[0050] c. apply second coat after 2 to 4 minutes;
[0051] d. apply third and further coats at 2 to 4 minute intervals until appearance of crystals or "frosting";
[0052] e. after appearance of crystals or "frosting", clean face with dist...
example 2
[0057] A therapeutic and prophylactic superficial peel in solution form may be prepared as follows:
[0058] L-lactic acid 14 grams, citric acid 14 grams, salicylic acid 14 grams, and kojic acid 2 grams are dissolved in a mixture of ethanol 40 ml and distilled water 16 ml. and stirred until a clear solution is obtained. The solution should be tightly capped until used.
example 3
[0059] Another therapeutic and prophylactic peel with the addition of the skin lightener hydroquinone may be prepared as follows:
[0060] L-lactic acid 14 grams, citric acid 14 grams, salicylic acid 14 grams, kojic acid 2 grams, and hydroquinone 1 gram are dissolved in a mixture of ethanol 39 ml and distilled water 16 ml. and stirred until a clear solution is obtained. This formulation is appropriate for age spots and keratoses.
PUM
| Property | Measurement | Unit |
|---|---|---|
| composition | aaaaa | aaaaa |
| strength | aaaaa | aaaaa |
| depth | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com