Gene therapy of diseases associated with the immune system, using a cell-specific active compound which is regulated by the cell cycle
a technology of immune system and gene therapy, applied in the direction of bacterial antigen ingredients, sugar derivatives, biochemistry apparatus and processes, etc., can solve the problems of transplanted organ rejection, chronic infections, poor vaccination,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
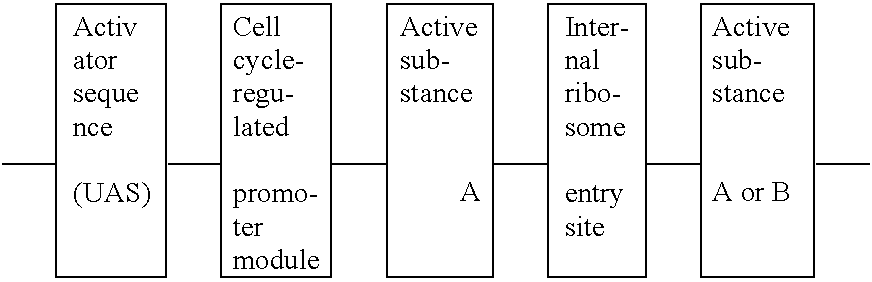
[0083] An activator sequence (UAS=upstream activator sequence) is to be understood to be a nucleotide sequence (promoter sequence or enhancer sequence) with which transcription factors, which are formed or are active in the target cell, interact. The CMV enhancer, the CMV promoter (EP 0173. 177.B1), the SV40 promoter, or any other promoter sequence or enhancer sequence known to the skilled person, can be used as an activator sequence.
[0084] Within the meaning of this invention, however, the preferred activator sequences include gene-regulatory sequences or elements from genes which encode proteins which are formed, in particular, in cells of the hematopoietic system, in activated lymphocytes, in activated synovial cells or macrophages, in virus-infected cells or in leukemia cells.
4.3. Description of the Active Substance
[0085] The active substance is to be understood to be the DNA for a protein which is to bring about the therapeutic effect, i.e. the curing of the disease of the immu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| colloidal dispersion | aaaaa | aaaaa |
| membrane structures | aaaaa | aaaaa |
| area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com