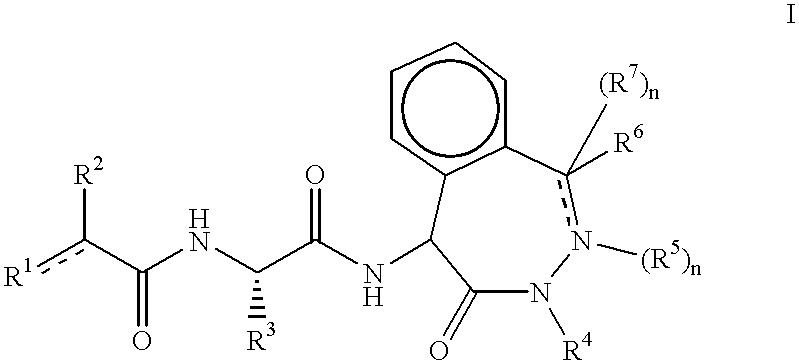
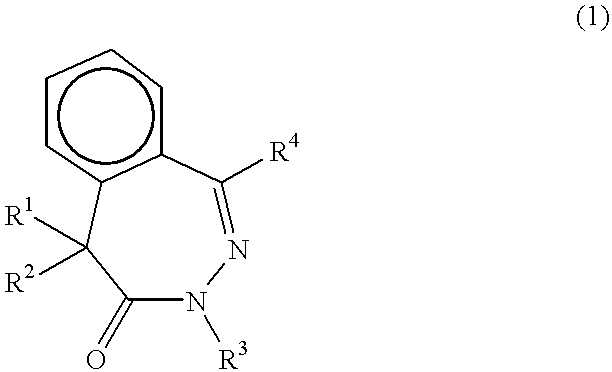
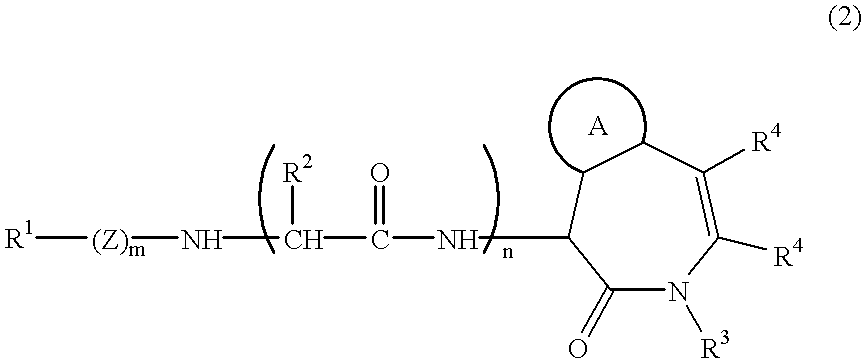
Benzodiazepinone beta -amyloid inhibitors: arylacetamidoalanyl derivatives
a technology of arylacetamidoalanyl and benzodiazepine, which is applied in the direction of biocide, peptide/protein ingredient, peptide/protein ingredient, etc., can solve the problems of increased alzheimer's disease cost, increased risk of adverse effects, so as to achieve effective anti-amyloid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
1-Methyl-2-(1-methylethyl)hydrazine (VII)
[0064] a) To the stirred solution of N-methyl hydrazine (25.00 g, 543.47 mmol ) in THF (600 ml) at -10.degree. C. was added di-tert-butyl dicarbonate (124.5 g, 167 mmol). The resulting mixture was stirred for 1 h and diluted with ethyl acetate (400 ml), further washed with water (1 L), brine (600 ml) and dried over magnesium sulfate. Filtration and concentration in vacuo produced 70 g of crude 1-methylhydrazinecarboxylic acid, 1,1 -dimethylethyl ester.
[0065] b) To the solution of above compound (70 g, 479.45 mmol) in ether (1 L) was added acetone (46.34 mL), 3 mL of acetic acid and 500 mg of sodium acetate. The solution was stirred further for an hour and was then concentrated. The resulting residue was diluted with ethyl acetate (800 mL) and washed with water (700 mL), brine (700 mL), dried over magnesium sulfate, filtrated and concentrated to afford 80 g of 2-propanone, methyl-(1,1-dimethylethoxycarbonyl)hydrazone.
[0066] c) To lithium alumi...
example 2
2,3,4,5-Tetrahydro-3-methyl-2-(1-methylethyl)-1 H-2,3-benzodiazepin-1,4-di-one (VIA)
[0068] To the solution of homophthalic anhydride (6.80 g, 41.9 mmol) in 70 mL of glacial acetic acid was added 1-methyl-2-(1-methylethyl)hydrazine, dihydrochloride (6.75 g, 41.9 mmol) in 35 mL of pyridine (Rosen, et al., J. Heterocyclic Chem., 1969, 6, 9-12). The solution was refluxed for 12 h, cooled, diluted with water (300 mL), and extracted with chloroform (3.times.400 mL). The chloroform extract was washed with 10% hydrochloric acid (600 mL) and 5% sodium bicarbonate (600 mL) , dried over magnesium sulfate and concentrated to dryness. The mixture was purified by silica gel chromatography (hexane / ethyl acetate, 3 / 1) to afford 2,3,4,5-tetrahydro-3-methyl-2-(1-methylethyl)-1H-2,3-benzodiazepin-1,4-di-one (5 g, 51%).
example 3
5-(R,S)-Azido-2,3,4,5-tetrahydro-3-methyl-2-(1-methylethyl)-1 H-2,3-benzodiazepin-1,4-dione (VA)
[0069] Potassium bis(trimethylsilyl)amide (6.54 g, 32.8 mmol) was added to a -78.degree. C. solution of above compound (3.8 g, 16.3 mmol) in THF (200 mL) followed by the addition of trisyl azide (5.49 g, 17.7 mmol) solution in THF (5 mL) at -78.degree. C. via cannula after 5 minutes (Evans, et al., J. Amer. Chem. Soc. 1990, 112, 4011-4030; Butcher, et al., Tetrahedron Lett., 1996, 37 (37), 6685-6688). The reaction mixture was further stirred for another 5 min at -78.degree. C., and 4.5 mL of glacial acetic acid was added and the reaction mixture was warmed to 30.degree. C. over a period of 2 h. Aqueous sodium bicarbonate (200 mL) was added and the mixture was extracted with dichloromethane (3.times.300 mL). The organic layer was washed with brine (600 mL) dried over magnesium sulfate and concentrated. The resulting solid was purified on silica gel column to afford 5-azido-2,3,4,5-tetrahyd...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| flow rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com