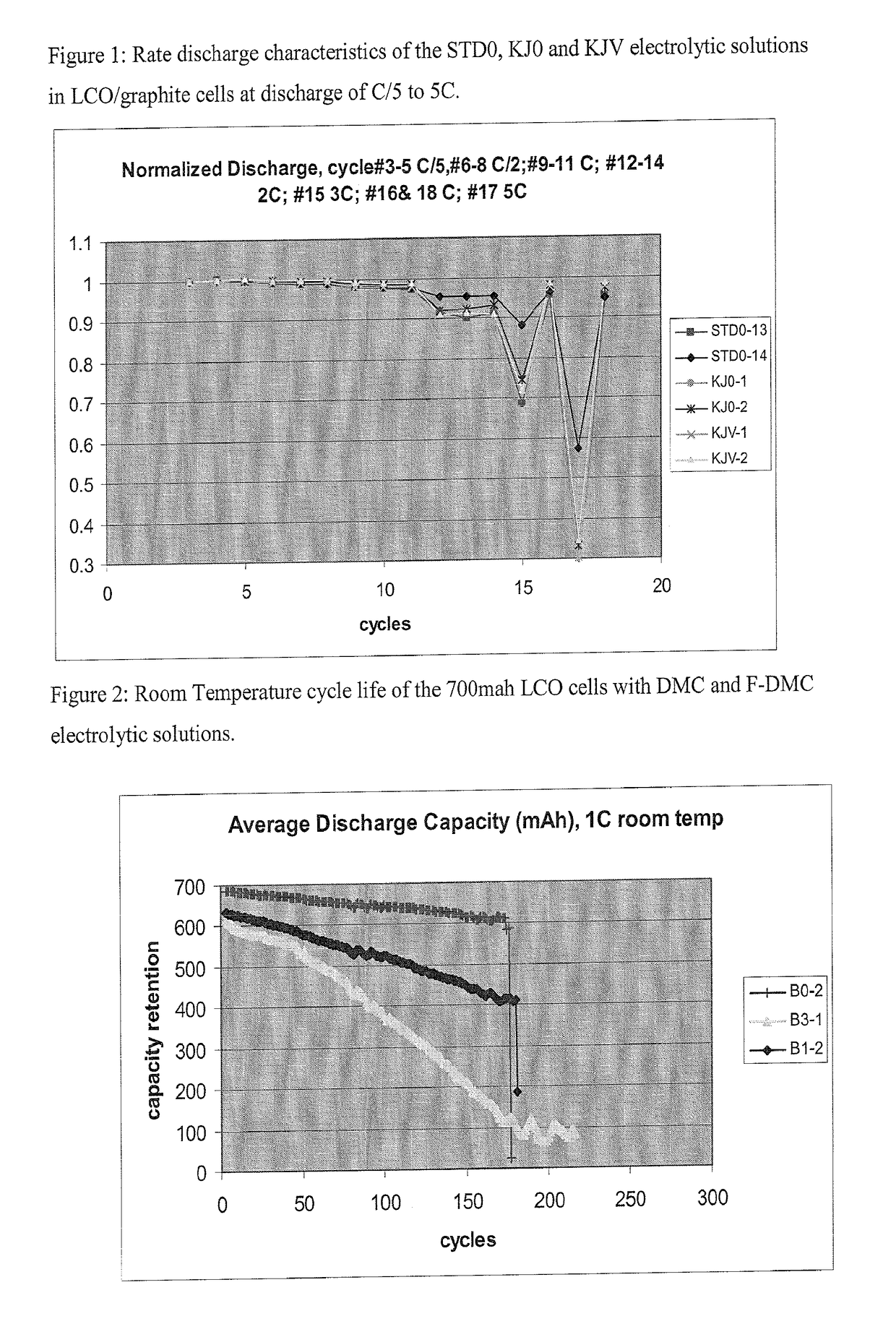
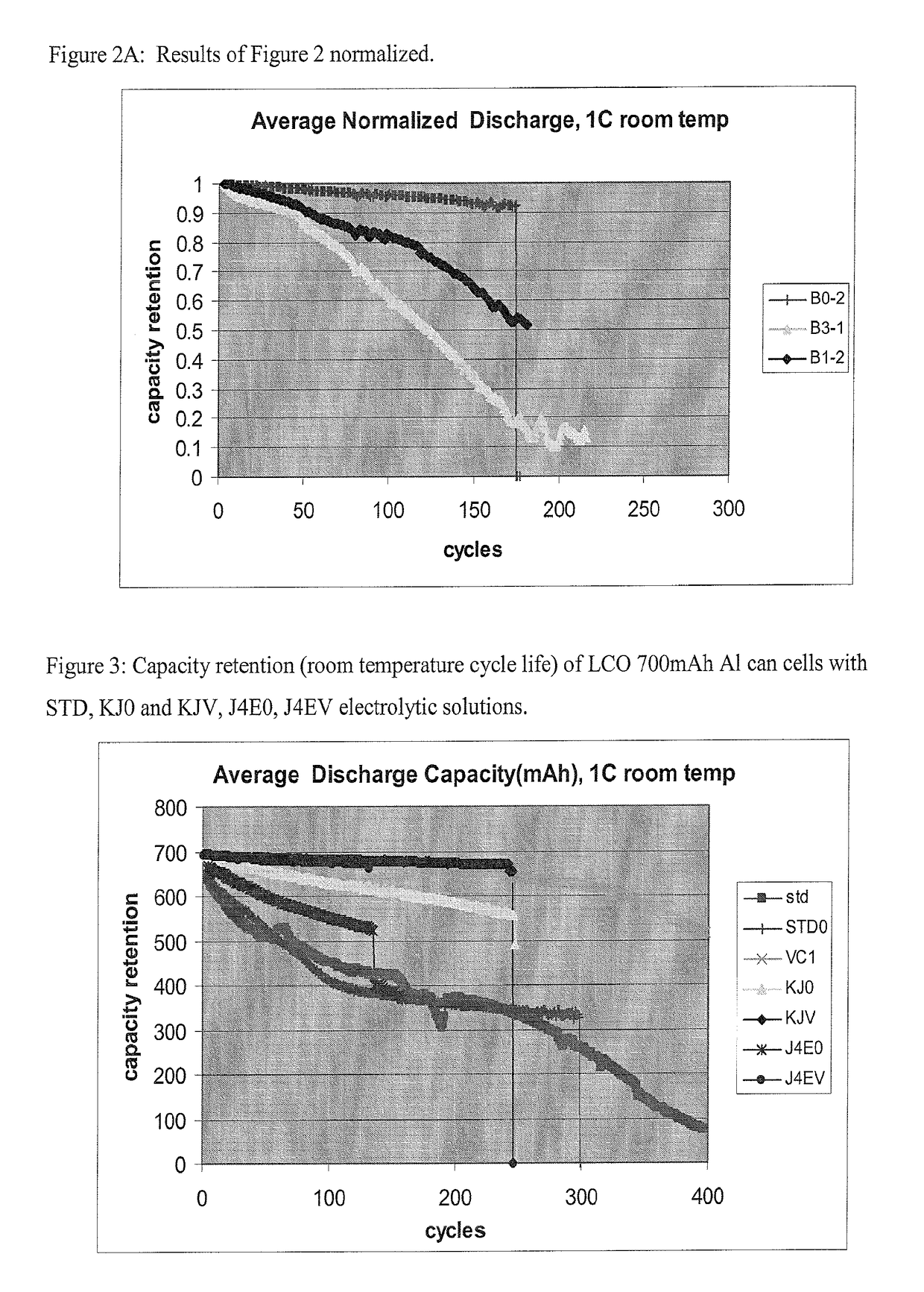
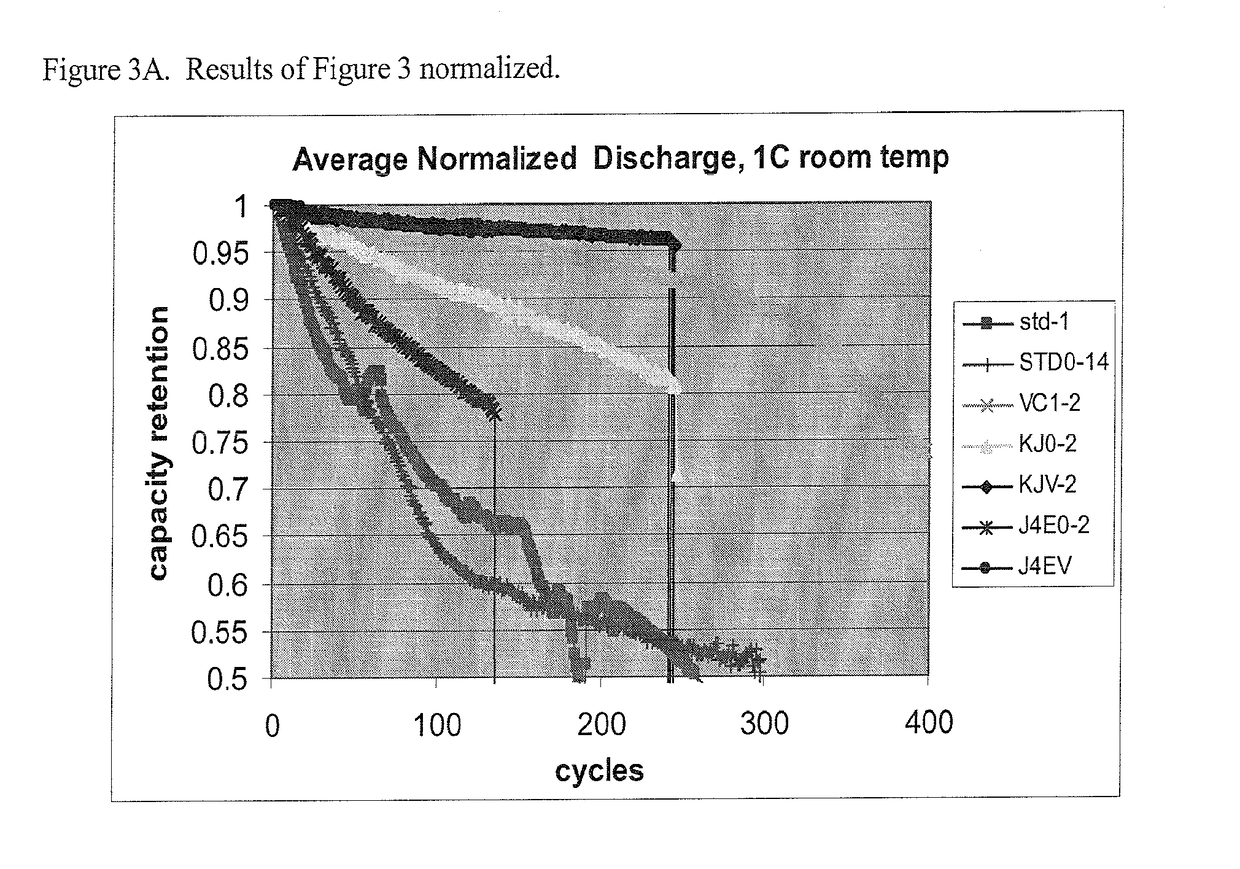
Non-aqueous electrolytic solutions and electrochemical cells comprising the same
a technology of electrolysis solution and electrochemical cell, which is applied in the manufacture of final products, basic electric elements, electrochemical generators, etc., can solve the problems of low conductivity, low efficiency, and high flammability of electrolytic solutions currently used in lithium or lithium ion batteries. achieve the effect of distributing non-flammability to the electrolyti
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0024]The following embodiments describe the preferred mode presently contemplated for carrying out the invention and are not intended to describe all possible modifications and variations consistent with the spirit and purpose of the invention. Features and advantages of the present invention will become more readily apparent to those skilled in the art upon consideration of the following detailed description.
[0025]More precisely, the invention relates to an electrolytic solution comprising a lithium salt and a solvent, (a) wherein the solvent comprises (i) at least one of a cyclic carbonate and a linear carbonate, (ii) at least one phosphazene compound represented by the structure (PNX1X2X3X4X5)n, where X1-X5 are substituents that are independently selected from the group consisting of nothing, halogen, oxygen, sulfur, alkyl, alkoxyl, phenyl, phenoxyl, or siloxyl, with the proviso that at least one substituent X is present, and is / or contains fluorine, and n is 1-4, (iii) at least...
PUM
| Property | Measurement | Unit |
|---|---|---|
| vol % | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| constant current rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com