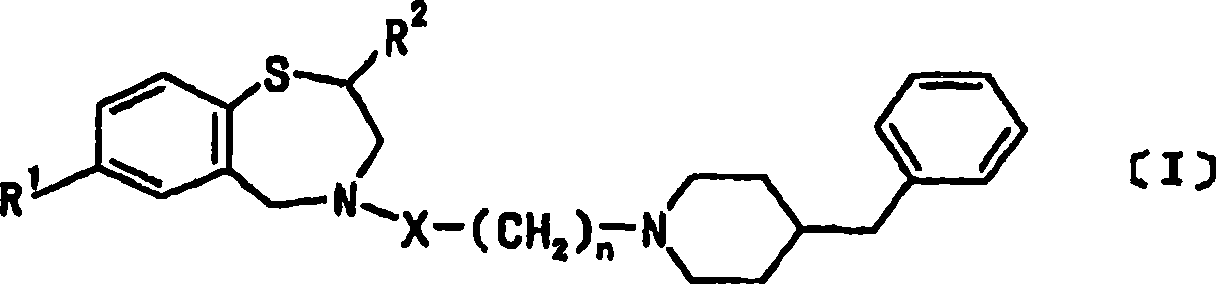Muscle relaxation accelerator and therapeutic agent for muscular tissue diseases such as muscle relaxation failure
A relaxation, muscle technique, used in the treatment or prevention of acute pulmonary edema, heart failure treatment or prevention, active compounds, angina pectoris, catecholamine-induced hypertension treatment, myocardial diastolic disorders Related diseases, drugs for the treatment of hypertension, drugs for the treatment or prevention of intramyocardial microvascular angina pectoris, drugs for the treatment or prevention of catecholamine-induced arrhythmia, can solve the problems of decreased blood drug concentration, coronary perfusion disorder, and failure to prevent sudden death, etc. , to improve blood flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
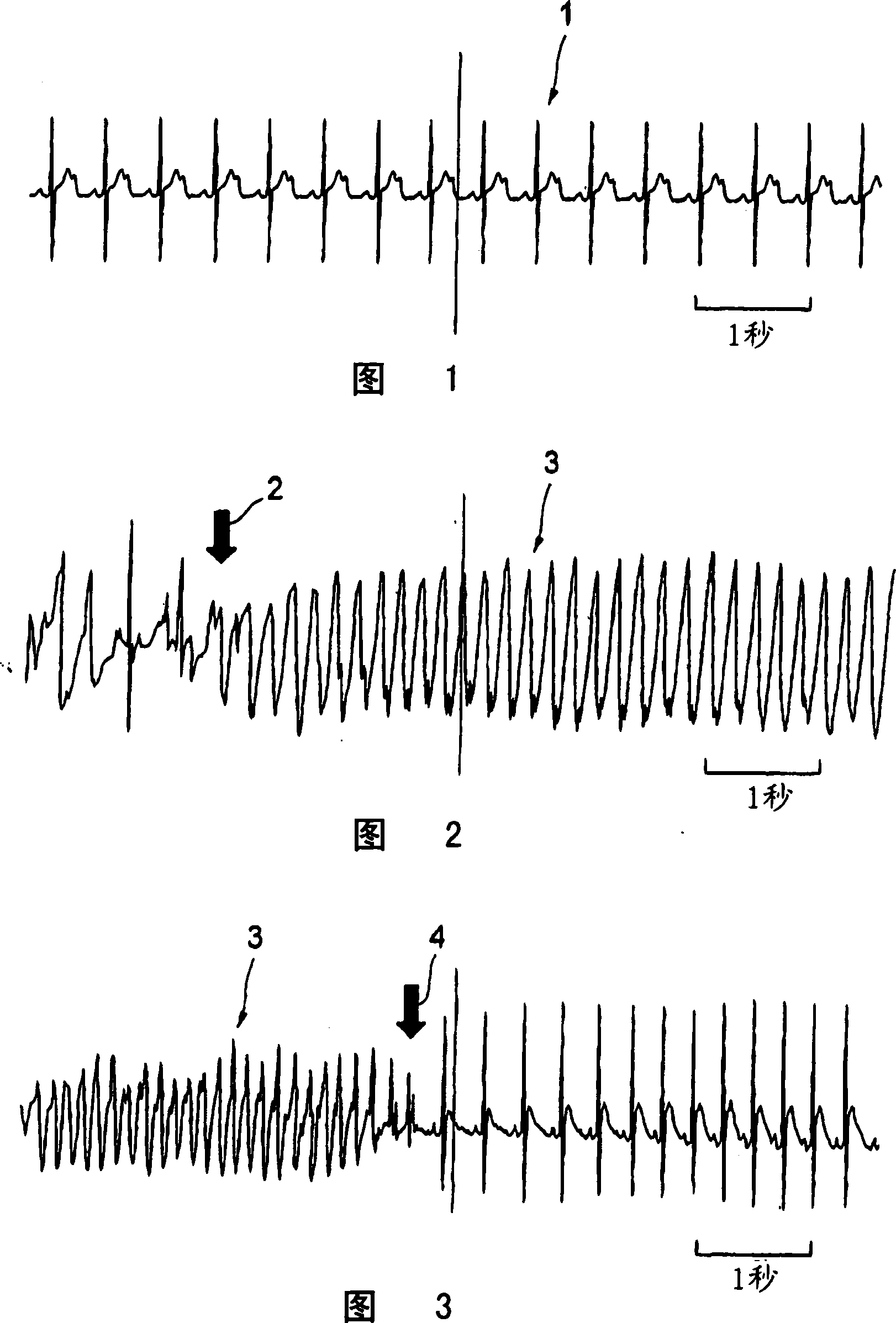
[0057] In this example, the pharmaceutically acceptable salt of the above-mentioned 1,4 benzothiazepine derivatives uses the monohydrochloride of this compound, that is, 4-[3-(4-benzylpiperidin-1-yl)propionyl ]-7-methoxy-2,3,4,5-tetrahydro-1,4-benzothiazepine monohydrochloride (hereinafter referred to as the present compound). Wistar male rats, 8 weeks old and weighing 300-330 g, were used. Anesthesia was performed by subperitoneal injection of 1000 mg / kg urethane and 80 mg / kg α-chloralose, and natural respiration was used. In this example, 100 mg of the present compound was dissolved in 1 mL of dimethyl sulfoxide (DMSO) to prepare a DMSO solution of the present compound, and the solution was stored at 4°C. The injection amount of norepinephrine is 40 μg / kg / min, and its preparation method is to dissolve 1 mg of norepinephrine in 41 μL of distilled water to make a norepinephrine solution.
[0058] First, a cannula for continuous infusion of calcium chloride aqueous solution o...
experiment example 2
[0069] The effect of this compound on blood pressure
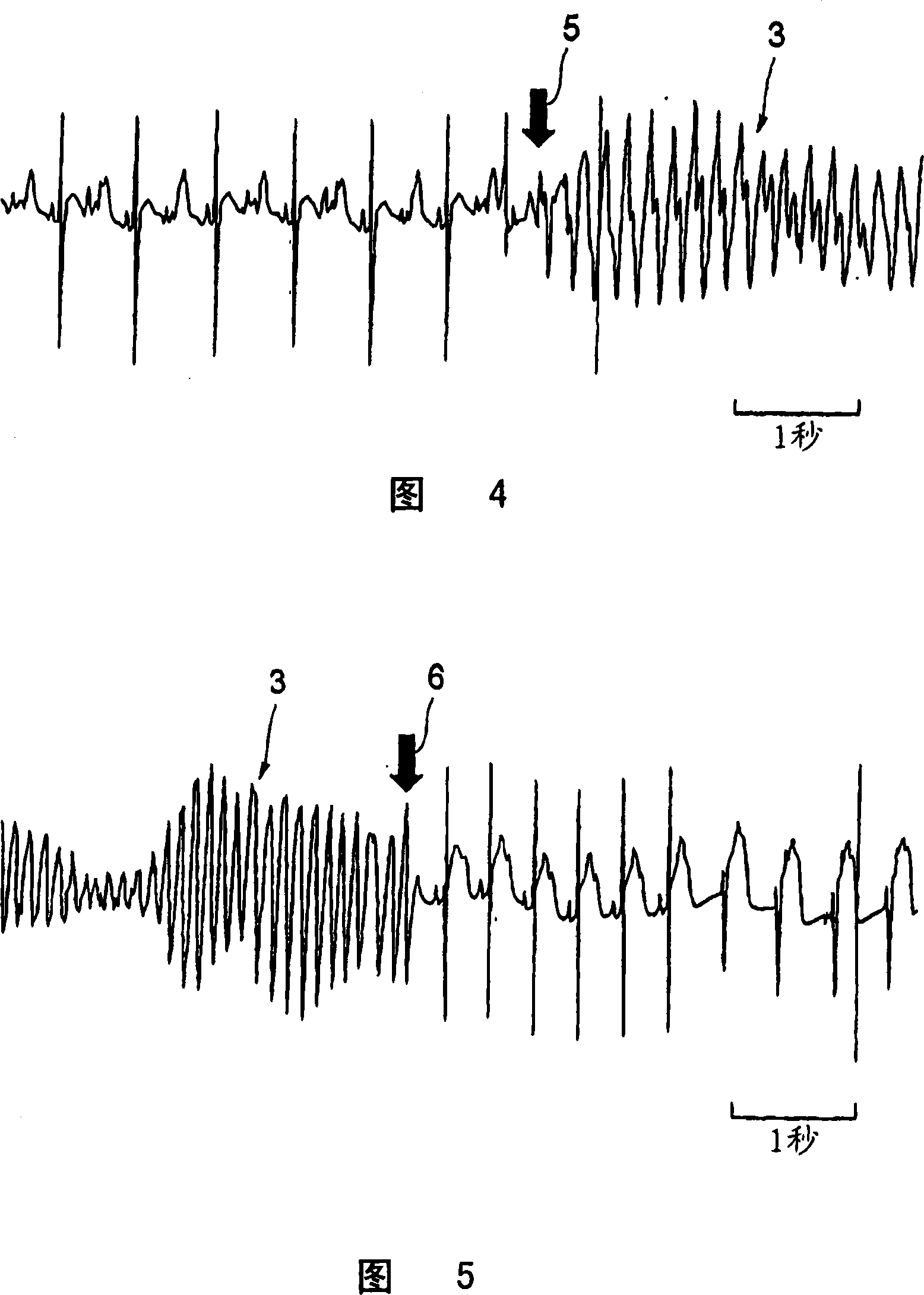
[0070] In this experiment, 8-week-old wistar male rats weighing 310 and 330 g were used. Anesthesia was performed by subperitoneal injection of 1000 mg / kg urethane and 80 mg / kg α-chloralose, and natural respiration was used. In this experimental example, 100 mg of the present compound was dissolved in 1 mL of dimethylsulfoxide (DMSO) to prepare a DMSO solution of the present compound, and the solution was stored at 4°C. Separately, 1 mg of norepinephrine was dissolved in 41 μL of distilled water to prepare a norepinephrine solution.
[0071] This example was carried out at a room temperature of 20-25° C. as in the above-mentioned Experimental Example 1. This example is also the same as the above-mentioned Experimental Example 1. The cannula that continuously injects calcium chloride aqueous solution or norepinephrine aqueous solution containing calcium chloride is inserted into the right external jugular vein of the abov...
experiment example 3
[0083] Study on the effect of the compound on the diastolic ability of the left ventricular wall by tissue Doppler method
[0084] In this experimental example, 9-week-old Wistar male rats with body weights of 310 and 320 g were used. Anesthesia was performed by subperitoneal injection of 1000 mg / kg urethane and 80 mg / kg α-chloralose, and natural respiration was used. In this experimental example, 100 mg of the present compound was dissolved in 1 mL of dimethyl sulfoxide (DMSO) to prepare a DMSO solution of the present compound, and the solution was stored at 4°C. Separately, 1 mg of norepinephrine was dissolved in 41 μL of distilled water to prepare a norepinephrine solution.
[0085] This example was carried out at a room temperature of 20-25° C. as in the above-mentioned Experimental Example 1. This example is also the same as the above-mentioned Experimental Example 1. A cannula that continuously injects calcium chloride aqueous solution or norepinephrine aqueous solutio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com