Nickel zinc ferrite material and its preparation method
A technology of nickel-zinc ferrite and nickel nitrate, which is applied in the field of nickel-zinc ferrite materials and its preparation, can solve the problems of high energy consumption and achieve the effects of low energy consumption, short synthesis time, and uniform particle size distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
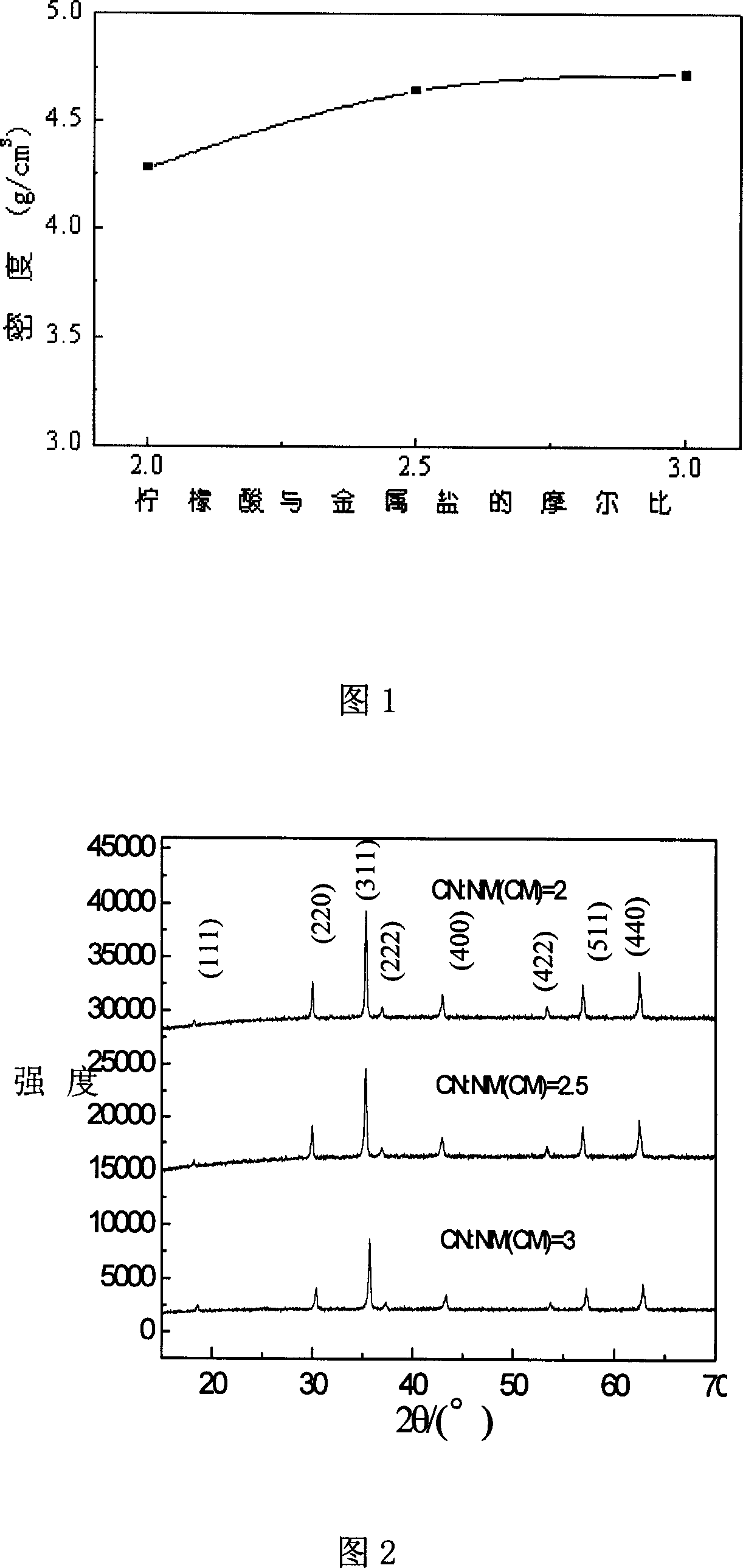
[0020] Press Ni 0.5 Zn 0.5 Fe 2 o 4 The stoichiometric ratio takes analytically pure ferric citrate, zinc nitrate and nickel nitrate, dissolves ferric citrate, nickel nitrate and zinc nitrate in deionized water respectively, and adds analytically pure citric acid after mixing, the molar ratio of citric acid to ferric citrate Respectively 2:1, 2.5:1, 3:1, stir evenly to obtain a clear precursor solution; heat the precursor solution to evaporate the solvent to form a black-red foamy substance, which is self-combusting, and the foamy substance expands to obtain Loose brown powder; press the brown powder into a disc with a diameter of 1.8 cm and a thickness of 1.5 mm under a pressure of 5 MPa, and sinter at 1300 ° C for 2 hours.
[0021] The relative density test results of the samples are shown in Figure 1. It can be seen from the figure that when the molar ratio of citric acid to ferric citrate is 3:1, the density of the sample is 4.75, and when the molar ratio of citric aci...
Embodiment 2
[0023] Press Ni 0.5 Zn 0.5 Fe 2 o 4 The stoichiometric ratio takes analytically pure ferric citrate, zinc nitrate and nickel nitrate, dissolves ferric citrate, nickel nitrate and zinc nitrate in deionized water respectively, and adds analytically pure citric acid after mixing, the molar ratio of citric acid to ferric citrate Respectively 2:1, 2.5:1, 3:1, stir evenly to obtain a clear precursor solution; heat the precursor solution to evaporate the solvent to form a black-red foamy substance, which is self-combusting, and the foamy substance expands to obtain Loose brown powder; press the brown powder into a disc with a diameter of 1.8 cm and a thickness of 1.5 mm under a pressure of 7 MPa, and sinter at 700 ° C for 3 hours. The phase structure was analyzed by X-ray diffraction (XRD), and the test results are shown in Figure 2. It can be seen from Figure 2 that the structures of the samples obtained with different amounts of citric acid are all single spinel structures.
Embodiment 3
[0025] Press Ni 0.5 Zn 0.5 Fe 2 o 4 The stoichiometric ratio takes analytically pure ferric citrate, zinc nitrate and nickel nitrate, dissolves ferric citrate, nickel nitrate and zinc nitrate in deionized water respectively, and adds analytically pure citric acid after mixing, the molar ratio of citric acid to ferric citrate Respectively 2:1, 2.5:1, 3:1, stir evenly to obtain a clear precursor solution; heat the precursor solution to evaporate the solvent to form a black-red foamy substance, which is self-combusting, and the foamy substance expands to obtain Loose brown powder; press the brown powder into a disc with a diameter of 1.8 cm and a thickness of 1.5 mm under a pressure of 10 MPa, and sinter at 1100 ° C for 5 hours. The sintered surface of the sample was studied with a field emission scanning electron microscope, and the test results are shown in Figure 3 (a), (b), (c). Figure (a) is the SEM figure of the molar ratio of citric acid and ferric citrate being 2:1 re...
PUM
| Property | Measurement | Unit |
|---|---|---|
| dielectric loss | aaaaa | aaaaa |
| electrical resistivity | aaaaa | aaaaa |
| dielectric loss | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com