Method for preparing hollow fiber with temperature sensitive medicinal hydrogel and application thereof
A technology of temperature-sensitive hydrogel and temperature-sensitive gel, which is applied in the manufacture of hollow filaments, pharmaceutical formulations, fiber chemical characteristics, etc., to achieve the effect of maintaining blood drug concentration, stabilizing blood drug concentration, and alleviating pain and fear
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
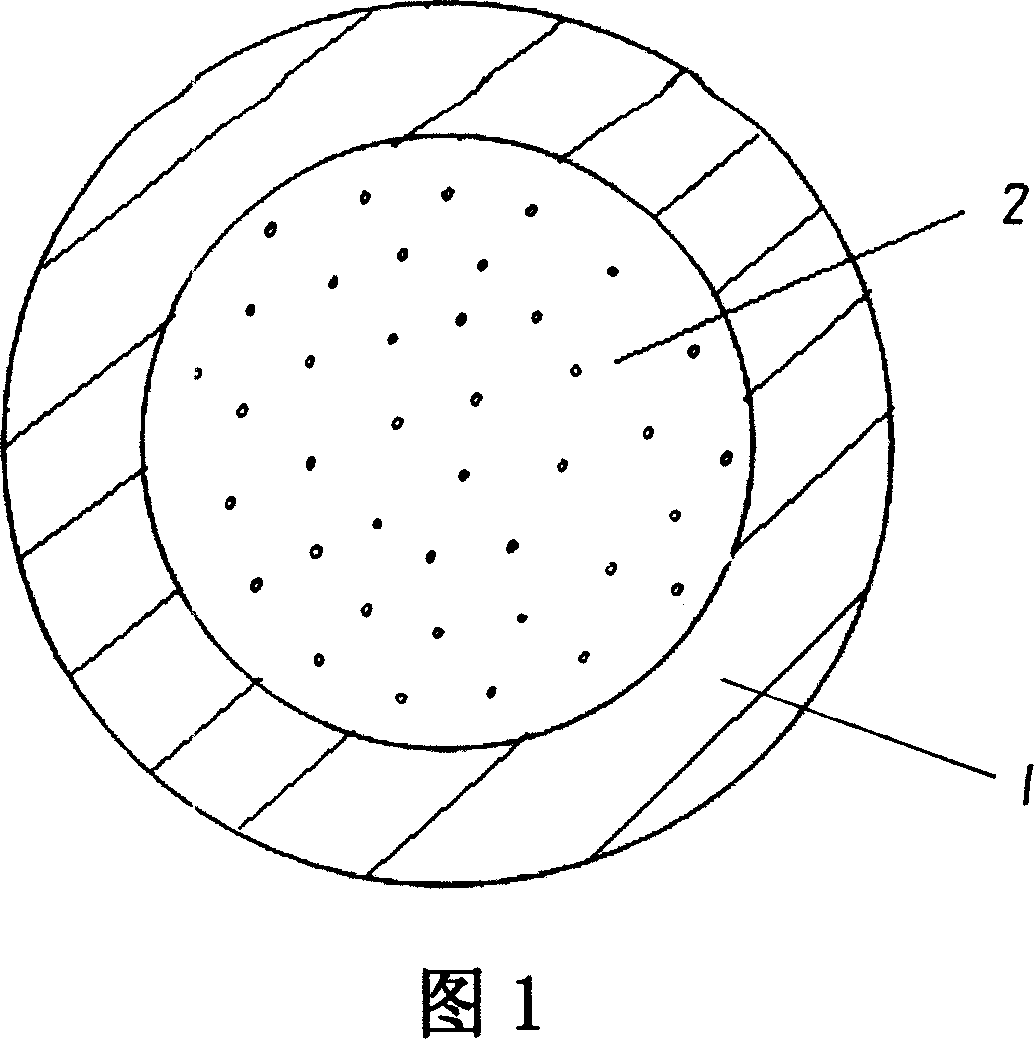
[0032] A drug temperature-sensitive hydrogel hollow fiber, with the hollow fiber 1 as a carrier, wherein, the inner cavity of the hollow fiber 1 contains a drug temperature-sensitive hydrogel 2, which is a kind of temperature-sensitive hydrogel that will A phase transition occurs and the drug is released from the hydrogel.
[0033] A method for preparing a drug thermosensitive hydrogel hollow fiber, wherein the drug thermosensitive hydrogel 2 is synthesized in the cavity inside the hollow fiber 1, including the following process:
[0034] The specific process is as follows:
[0035] (1) Preparation and treatment of hollow fibers: polyacrylonitrile hollow fibers were spun, and soaked in pure water for 24 hours after pore-preserving treatment to ensure that the surface of the membrane pores was completely infiltrated by water.
[0036] (2) Insertion of temperature-sensitive monomer solution: prepare 300ml of N-isopropylacrylamide (NIPA) solution with a concentration of 1mol / L, ...
Embodiment 2
[0042] A drug temperature-sensitive hydrogel hollow fiber, with the hollow fiber 1 as a carrier, wherein, the inner cavity of the hollow fiber 1 contains a drug temperature-sensitive hydrogel 2, which is a kind of temperature-sensitive hydrogel that will A phase transition occurs and the drug is released from the hydrogel.
[0043] The preparation method of the drug thermosensitive hydrogel hollow fiber, the drug thermosensitive hydrogel 2 is synthesized in the cavity inside the hollow fiber 1, including the following process:
[0044] (1) Preparation of hollow fibers: Spin cellulose acetate hollow fibers, soak them in pure water for 24 hours after pore-preserving treatment, to ensure that the surface of membrane pores is completely infiltrated by water.
[0045] (2) Insertion of temperature-sensitive monomer solution: same as in Example 1.
[0046] (3) Synthesis of thermosensitive hydrogel: Same as Example 1.
[0047] (4) Preparation of the drug hydrogel: the extract contai...
Embodiment 3
[0051] A drug temperature-sensitive hydrogel hollow fiber, with the hollow fiber 1 as a carrier, wherein, the inner cavity of the hollow fiber 1 contains a drug temperature-sensitive hydrogel 2, which is a kind of temperature-sensitive hydrogel that will A phase transition occurs and the drug is released from the hydrogel. The preparation method of the drug thermosensitive hydrogel hollow fiber, the drug thermosensitive hydrogel 2 is synthesized in the cavity inside the hollow fiber 1, including the following process:
[0052] (1) Preparation of hollow fiber: polyester hollow fiber is spun, soaked in pure water for 24 hours after pore-preserving treatment, to ensure that the membrane pore surface is completely infiltrated by water.
[0053] (2) Insertion of temperature-sensitive monomer solution: same as in Example 1.
[0054] (3) Synthesis of thermosensitive hydrogel: Same as Example 1.
[0055] (4) Preparation of drug hydrogel: mifepristone was used as drug. By volume rat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com