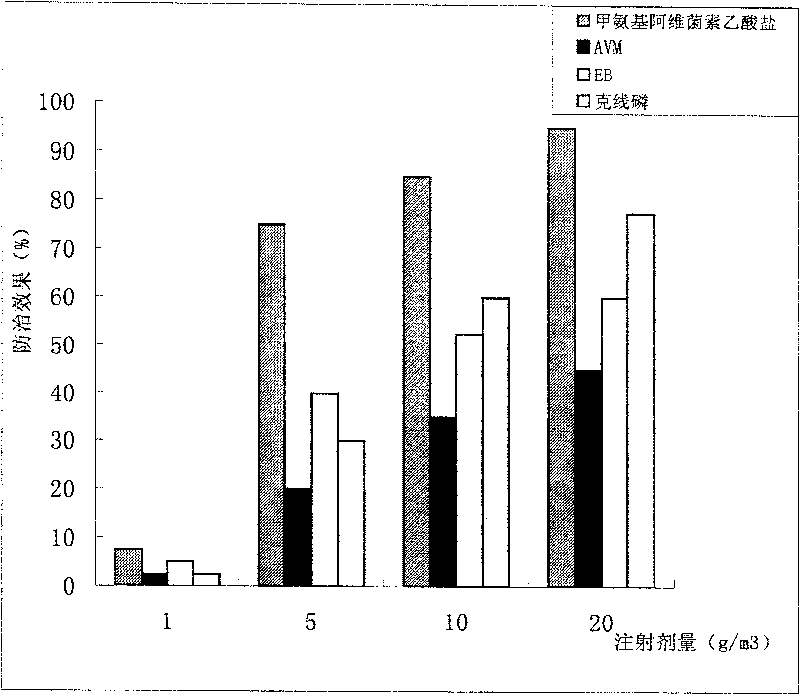
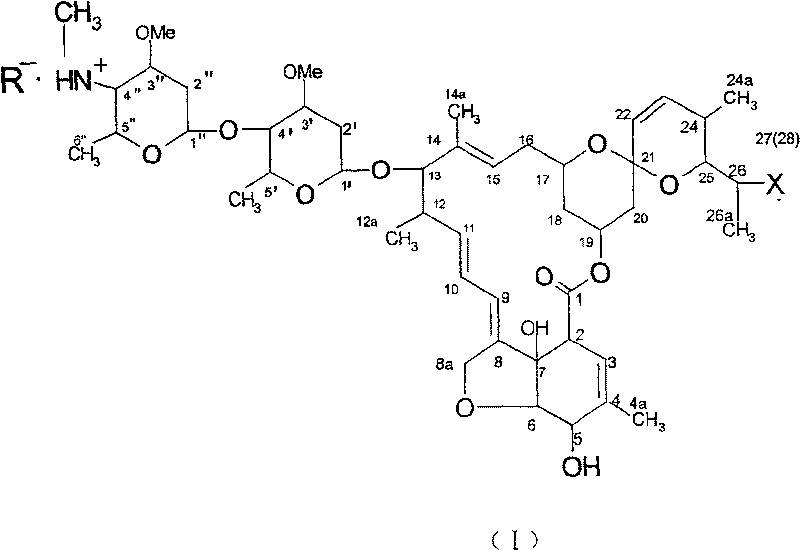
Methyl amine abamectin acylate and its preparation method and application
A technology of methylamino abamectin and organic acid salt, applied in the field of pesticides, can solve the problems of low activity, restricted drug application, narrow insecticidal spectrum, etc., and achieves the effect of good insecticidal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Accurately weigh 10 g of distilled water into a 10 ml graduated test tube with an electronic analytical balance, and place it in a water bath at 25°C. Then each compound was added thereto, and the mixture was shaken until it could no longer be dissolved. Repeat 3 times. After wiping off and weighing, the obtained results of the solubility of the substance in water can be calculated in Table 1. From Table 1, we can see that, compared with emamectin benzoate, the solubility of emamectin organic acid salt in water has increased by nearly tens to hundreds of times.
[0029] The solubility of table 1 emamectin organic acid salt in water
[0030]
[0031]
Embodiment 2
[0033] Under indoor conditions, the 3rd instar diamondback moth larvae were treated with leaf dipping method, and the LC of various pesticides on diamondback moth at 48 hours 50 See Table 2 for the values. As can be seen from Table 2, the activity of emamectin acetate to Plutella xylostella is much higher than that of other control agents, which are 4.52 times that of emamectin benzoate, 15.73 times that of abamectin, and that of profenofos. 335.81 times, 364.26 times of cyfluthrin, showing extremely strong insecticidal activity.
[0034] Table 2 The toxicity test results (48h) of pesticides such as emamectin acetate to the 3rd instar larvae of diamondback moth
[0035]
Embodiment 3
[0037] Under indoor conditions, the 3rd instar cotton bollworm larvae were treated by leaf dipping method, and the LC of various pesticides on cotton bollworm at 48 hours 50 See Table 3 for the values. As can be seen from Table 3, the activity of emamectin formate to cotton bollworm is much higher than that of other control agents, which are 4.29 times that of emamectin benzoate, 34.93 times that of abamectin, and 205.21 times that of cypermethrin. times, 115.82 times that of chlorchongdan, showing extremely strong insecticidal activity.
[0038] Table 3 Toxicity determination results (48h) of pesticides such as emamectin formate to the 3rd instar larvae of cotton bollworm
[0039]
[0040]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com