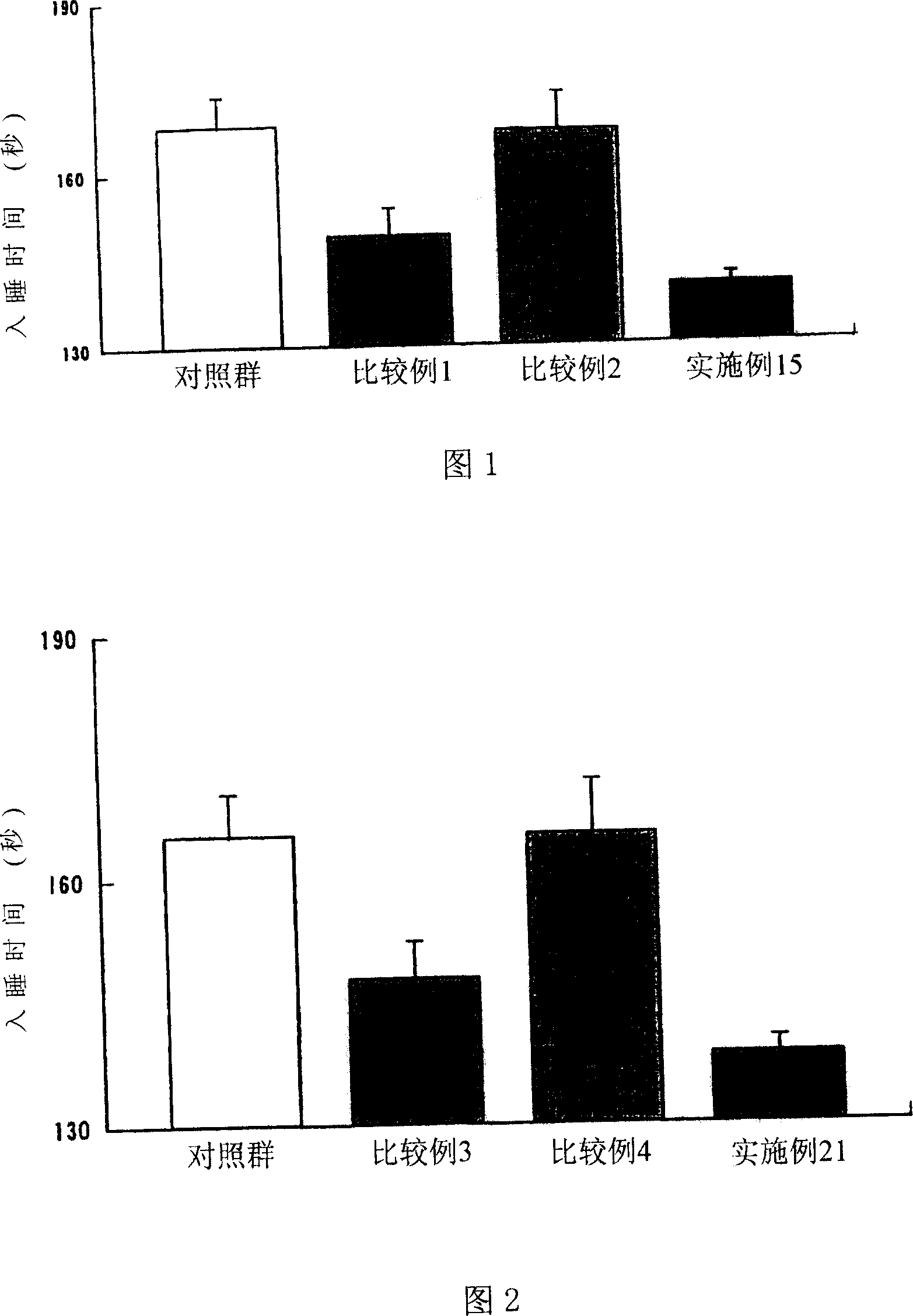
Medicine composition for improving sleep
A technology for improving sleep and composition, applied in the field of pharmaceutical composition for improving sleep, can solve problems such as insufficient effect, memory impairment, restlessness, etc., and achieve the effect of improving sleep effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0111] tablet:
[0112] Use 200 grams of dibenzamine hydrochloride, 90 grams of vine hook extract, 354 grams of lactose, 100 grams of corn starch, 302 grams of crystalline cellulose, 20 grams of cross-linked carmellose sodium (croscarmellose sodium), 24 grams of poly Vinylpyrrolidinone and 10 g of light anhydrous silicic acid were granulated by conventional methods to obtain granules for tableting. After 990 grams of the granules for tableting are mixed with 9 grams of talcum powder and 9 grams of magnesium stearate, the tablets are compressed to obtain 3300 plain tablets with a diameter of 9 millimeters, a thickness of 4.5 millimeters, and a weight of 280 milligrams.
Embodiment 2
[0114] tablet:
[0115] Use 200 grams of dibenzamine hydrochloride, 60 grams of hop extract, 384 grams of lactose, 100 grams of corn starch, 302 grams of crystalline cellulose, 20 grams of croscarmellose sodium, 24 grams of polyvinylpyrrolidone, 10 grams of light anhydrous silicic acid were granulated by conventional methods to obtain granules for tableting. After 990 grams of the granules for tableting are mixed with 9 grams of talcum powder and 9 grams of magnesium stearate, the tablets are compressed to obtain 3300 plain tablets with a diameter of 9 millimeters, a thickness of 4.5 millimeters, and a weight of 280 milligrams.
Embodiment 3
[0117] tablet:
[0118] Use 200 grams of dibenzamine hydrochloride, 60 grams of ginseng extract, 304 grams of lactose, 100 grams of corn starch, 382 grams of crystalline cellulose, 20 grams of croscarmellose sodium, 24 grams of polyvinylpyrrolidone, 10 grams of light anhydrous silicic acid were granulated by conventional methods to obtain granules for tableting. After 990 grams of the granules for tableting are mixed with 9 grams of talcum powder and 9 grams of magnesium stearate, the tablets are compressed to obtain 3300 plain tablets with a diameter of 9 millimeters, a thickness of 4.5 millimeters, and a weight of 280 milligrams.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com