Preparation method for optical enantiomer of ornidaxole
A technology of ornidazole optics and enantiomers, which is applied in the field of preparation of ornidazole optical enantiomers, can solve problems that are not suitable for industrial production, and achieve the effects of simple method, easy-to-obtain raw materials, and strong stereospecificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
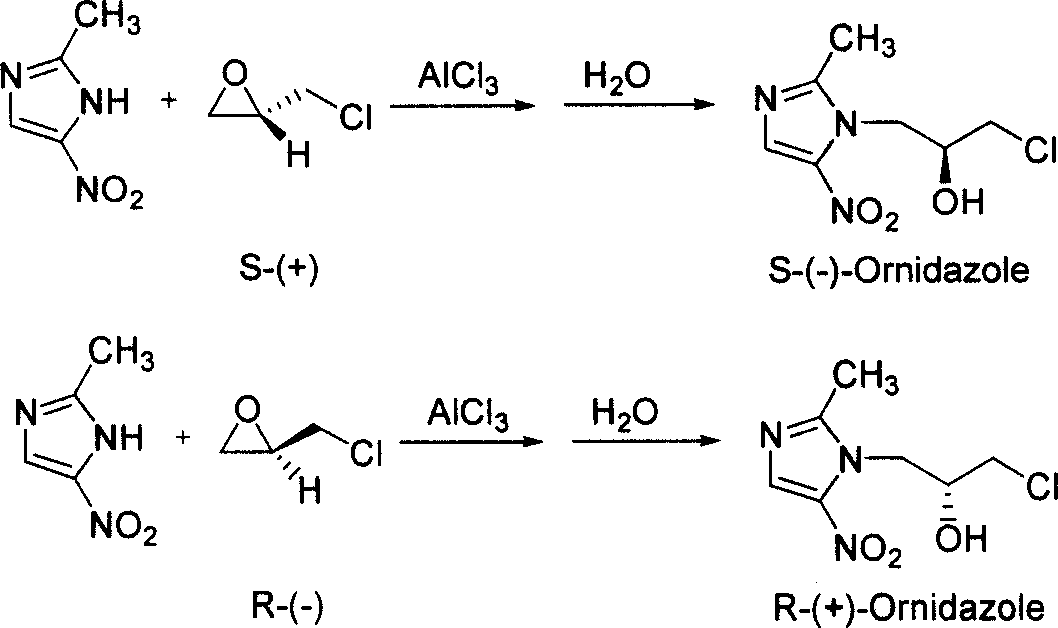
[0019] Preparation of S-(-)-ornidazole: Add 50.8 g (0.4 mol) of 2-methyl-5-nitroimidazole into 500 mL of ethyl acetate, and cool to 0-5°C. Add 80.1 g (0.6 mol) of anhydrous aluminum trichloride in batches, and control the reaction temperature not higher than 10°C. After the addition is complete, 55.5 g (0.6 mol) of S-(+)-epichlorohydrin is added dropwise to the reaction solution at 0-10°C, and the reaction is continued at 0-10°C for 4 hours after the drop is complete. After the reaction is complete, slowly add 200 mL of ice water to the reaction solution, control the temperature of the reaction solution to be lower than 30°C, stir for 1 hour, filter, add 50% sulfuric acid dropwise to the filtrate, adjust the pH to 1.0, and then add concentrated Adjust the pH to 3-4 with ammonia water, precipitate solids, filter, and adjust the pH of the obtained filtrate to 7.0-7.5 with concentrated ammonia water, separate the ethyl acetate layer, dry with anhydrous sodium sulfate, evaporate t...
Embodiment 2
[0022] Preparation of S-(-)-ornidazole: In 450 mL of dichloromethane, add 50.8 g (0.4 mol) of 2-methyl-5-nitroimidazole, and cool to 0-5°C. Add 80.1 g (0.6 mol) of anhydrous zinc chloride in batches, and control the reaction temperature not higher than 10°C. After the addition is complete, 55.5 g (0.6 mol) of S-(+)-epichlorohydrin is added dropwise to the reaction solution at 0-10°C, and the reaction is continued at 0-10°C for 6 hours after the addition is completed. After the reaction, 200 mL of ice water was slowly added to the reaction solution, the temperature of the reaction solution was controlled to be lower than 30° C., stirred for 1.5 hours, filtered, and the filtrate was added dropwise with 50% sulfuric acid to adjust the pH to 0.5. Then, dropwise added three Ethylamine was adjusted to pH 3-4, solid precipitated, filtered, and the resulting filtrate was adjusted to pH 7.0-7.5 with triethylamine, the dichloromethane layer was separated, dried with anhydrous magnesium ...
Embodiment 3
[0024] Preparation of R-(+)-ornidazole: Add 50.8 g (0.4 mol) of 2-methyl-5-nitroimidazole into 500 mL of ethyl acetate, and cool to 0-5°C. Add 80.1 g (0.6 mol) of anhydrous aluminum trichloride in batches, and control the reaction temperature not higher than 10°C. After the addition is complete, 55.5 g (0.6 mol) of R-(-)-epichlorohydrin is added dropwise to the reaction solution at 0-10°C, and the reaction is continued at 0-5°C for 5 hours after the drop is completed. After the reaction, slowly add 200 mL of ice water into the reaction liquid, control the temperature of the reaction liquid below 30°C, stir for 0.5 hours, filter, add hydrochloric acid dropwise to the filtrate, adjust the pH to 1.0, and then add concentrated ammonia water dropwise to the mixed liquid to adjust When the pH reached 3-4, solids were precipitated, filtered, and the obtained filtrate was adjusted to pH 7.0-7.5 with concentrated ammonia water, and the ethyl acetate layer was separated, dried with anhy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com