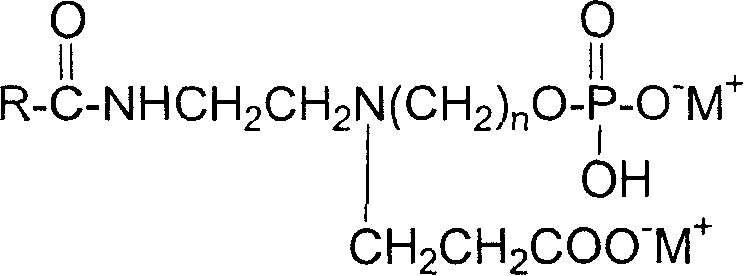Imidazoline amophoteric surface active agent and its synthesis technology
A technology of surfactant and imidazoline, which is applied in the field of imidazoline amphoteric surfactant and its synthesis process, to achieve excellent cleaning and detergency performance, high purity, and performance-enhancing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Melt 1 mol of lauric acid, add 1.1 mol of hydroxymethylethylenediamine, under the condition of residual pressure of 60mmHg and stirring, raise the temperature to 210°C at a rate of 3°C / min, and remove the reaction water in time, at this temperature Keep it for 1 hour, and then distill off excess hydroxymethylethylenediamine at a residual pressure of 10 mmHg to obtain an imidazoline intermediate. Add 1.2 mol of water and 0.1 mol of sodium hydroxide as a catalyst to the synthesized imidazoline intermediate, and react at 60° C. for 4 hours with sufficient stirring. After the imidazoline ring was completely hydrolyzed and opened, the temperature was raised to 110°C, and excess water was distilled off. After that, 1 mol of butyl acrylate was added dropwise, fully stirred and controlled at 70° C., and kept fully stirred and at the reaction temperature after the addition was completed, and the reaction was continued for 3 hours. Add 0.5 mol of phosphorus pentoxide in batches ...
Embodiment 2
[0030] Melt 1mol of palmitic acid first, add 1.3mol of hydroxyethylethylenediamine, under the condition of residual pressure of 70mmHg and stirring, raise the temperature to 220°C at a rate of 5°C / min, and remove the reaction water in time, at this temperature Keep it under the pressure for 1.5h, and then distill off excess hydroxyethylethylenediamine under the residual pressure of 15mmHg to obtain the imidazoline intermediate. Add 1.1 mol of water and 0.08 mol of potassium hydroxide as a catalyst to the synthesized imidazoline intermediate, and react at 80° C. for 2 hours under sufficient stirring. After the imidazoline ring is completely hydrolyzed and opened, the temperature is raised to 120°C, and excess water is distilled off. After that, 1 mol of ethyl acrylate was added dropwise, fully stirred and controlled at 60° C., and kept fully stirred and at the reaction temperature after the addition was completed, and the reaction was continued for 4 hours. Add 0.5 mol of phos...
Embodiment 3
[0032] First melt 1 mol of cocoic acid, add 1.5 mol of hydroxypropyl ethylenediamine, under the condition of residual pressure of 80mmHg and stirring, rise to 240 ℃ with a heating rate of 8 ℃ / min, and remove the reaction water in time. Keep at the temperature for 0.5h, and then distill off excess hydroxypropylethylenediamine at a residual pressure of 20mmHg to obtain an imidazoline intermediate. Add 1.4 mol of water and 0.07 mol of sodium hydroxide as a catalyst to the synthesized imidazoline intermediate, and react at 95° C. for 1 hour with full stirring. After the imidazoline ring is completely hydrolyzed and opened, the temperature is raised to 130°C, and excess water is distilled off. After that, 1 mol of propyl acrylate was added dropwise, fully stirred and controlled at 80° C., and kept fully stirred and at the reaction temperature after the addition was completed, and the reaction was continued for 2 hours. Add 0.5 mol of phosphorus pentoxide in batches to the reaction...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com