Targeting of ERB antigens
A technology of antigens and antibodies, applied in the direction of antibodies, instruments, analysis materials, etc., can solve problems such as competition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0151] Example 1: Conjugation and radiolabeling of trastuzumab
[0152] In this and subsequent examples, indium-111 was used in some instances in place of yttrium-90 because the former is a gamma-radiating source and has less radiation hazard than yttrium-90. The monoclonal antibody trastuzumab was mixed with 3-(13'-thioureabenzyl-DOTA)trioxadiamine-1-(13"-biotin-Asp-OH)trioxadiamine-5-iso Thiocyanate-aminoisophthalate (MitraTag TM -1033) (hereinafter also referred to simply as 1033) was conjugated using the method described by Wilbur D.S et al. in Bioconjuagte Chem. 13: 1079-1092, 2002. 10 mg of monoclonal antibody was dialyzed against 1 liter of metal-free HEPES at 4°C for 3 days with 3 buffer changes. Preparation of MitraTag TM Aqueous solution of -1033 (800μg), added to the antibody solution. After overnight incubation at room temperature, the antibody conjugate was dialyzed against 1 liter of metal-free 250 mM acetate buffer (pH 5.3) at 4° C. for 4 days with a minimum...
Embodiment 2
[0154] Example 2: Binding of 1033-conjugated trastuzumab to avidin adsorbent
[0155] Analysis of microcolumns bound to the avidin sorbent employed in the MitraDep(R) device 111 In-labeled 1033-trastuzumab radioconjugate fraction. About 97% of the radioactivity in the radiolabeled 1033-conjugate sample was bound to the microcolumn by the avidin sorbent.
Embodiment 3
[0156] Example 3: Affinity Analysis of Binding to Target Antigen
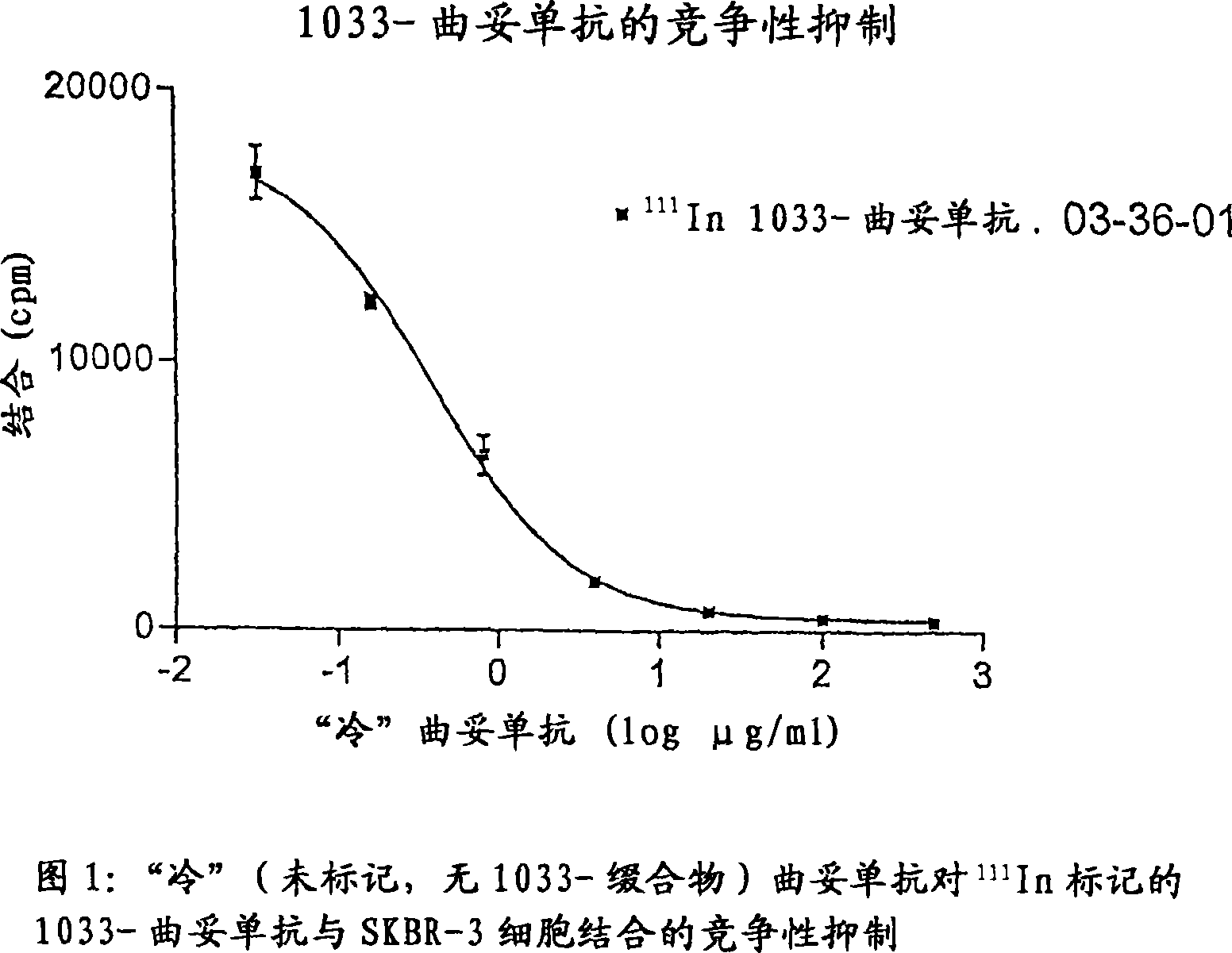
[0157] The effect of the conjugation process on the affinity (strength) of trastuzumab to the target antigen was studied using a competitive inhibition assay. Briefly, increasing amounts of trastuzumab were combined with constant amounts of 111 In-labeled 1033-trastuzumab mix. Add the mixture to fixed SK-BR3 cells in a 96-well plate. After 2 hours of incubation at room temperature, the wells were washed and the radioactivity bound to cells was measured in an automated NaI(T1) scintillation cytometer.
[0158] The amount of radioactivity bound was plotted against the concentration of trastuzumab ( figure 1 ), calculate the concentration required for 50% inhibition (IC 50 ). IC 50 is a measure of the relative affinity of the antibodies tested; a decrease in affinity is seen as the IC 50 Concentration increases. Significant changes in affinity usually indicate IC 50 The difference should be at least 10-fo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Dissociation constant | aaaaa | aaaaa |
| Dissociation constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com