Process for preparing piperidine derivative
A compound and chemical structure technology, applied in the field of preparation of piperidine derivatives, can solve the problems of not obtaining the target product, difficult product purification, cumbersome operation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
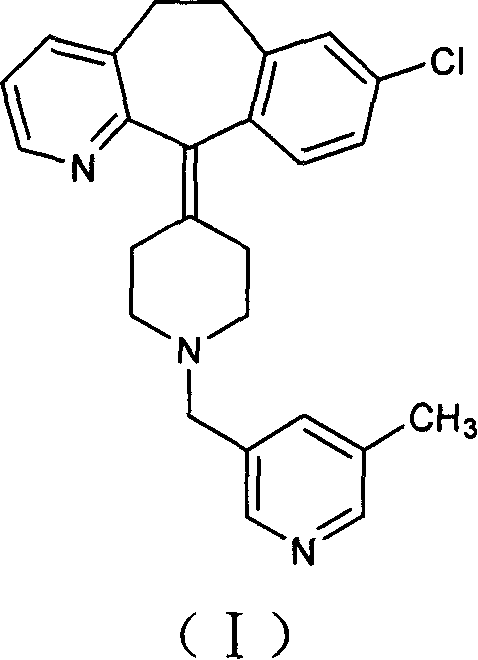
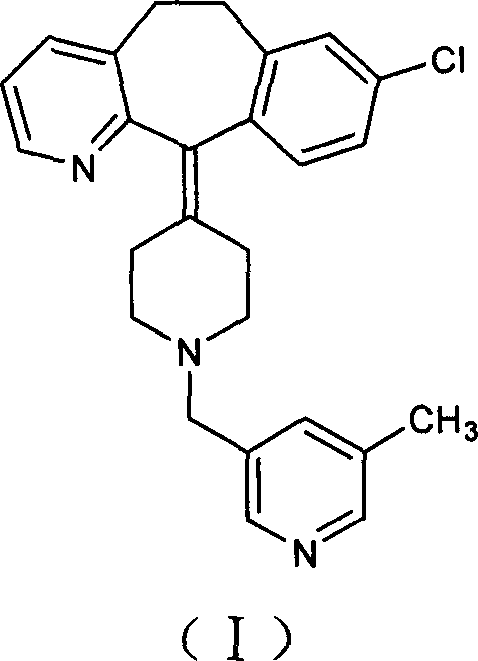
[0023] Under the protection of nitrogen, 150 ml of tetrahydrofuran and 50 ml of toluene were added to a 500 ml three-necked flask, and 15.0 g of vacuum-dried amide was added with stirring, and the temperature was lowered to 0° C. with an ice-salt bath. Take 18 milliliters of sodium dihydroaluminate (Red-Al), dilute it with 15 milliliters of toluene, add it dropwise into the reaction flask, after the addition is complete, stir for 36 hours. After the reaction was completed, 10 ml of water was added dropwise under an ice-water bath, stirred for 3 hours, filtered with suction, the filter cake was washed twice with tetrahydrofuran, and the filtrates were combined to remove the solvent under reduced pressure to obtain a brown-red viscous substance. Add toluene to dissolve, wash with dilute acid and dry. The desiccant was filtered off, the solution was precipitated, and solidified to obtain 8.7 g of the target compound. Yield: 60%.
[0024] HPLC purity: 98.5%.
Embodiment 2
[0026] Under the protection of nitrogen, 300 ml of tetrahydrofuran and 100 ml of toluene were added to a 1000 ml three-neck flask, 30.0 g of vacuum-dried amide was added under stirring, and the temperature was cooled to 0° C. with an ice-salt bath. Take 110 ml of sodium dihydroaluminate (Red-Al), dilute it with 100 ml of toluene, and add it dropwise into the reaction flask. After the dropwise addition was completed, the mixture was naturally raised to room temperature and stirred overnight. After the reaction was completed, 40 ml of water was added dropwise in an ice-water bath, stirred for 4 hours, filtered with suction, and the filter residue was washed twice with tetrahydrofuran, and the filtrates were combined to remove the solvent under reduced pressure to obtain a brown-red sticky substance. Add toluene to dissolve, wash with dilute acid and dry. The desiccant was filtered off, the solution was removed and solidified to obtain 20.2 g of the target compound. Yield: 70%....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com