Application of human sDR5 protein as medicine to treat virus hepatitis B
A viral hepatitis and therapeutic drug technology, applied in the application field of human sDR5 protein as a therapeutic drug for hepatitis B, to achieve the effect of reducing liver cell apoptosis, reducing liver cell damage and improving prognosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
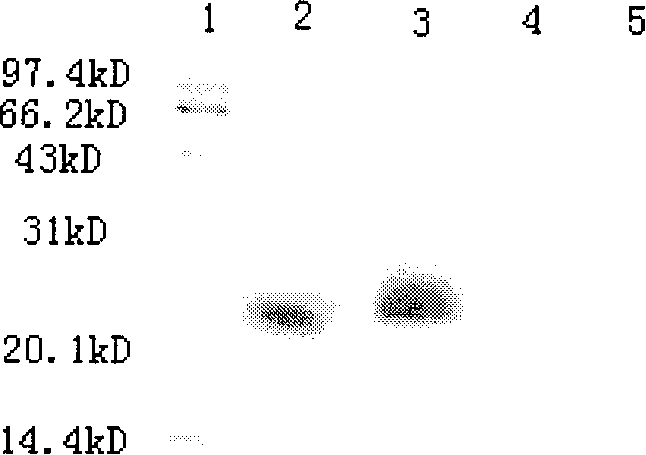
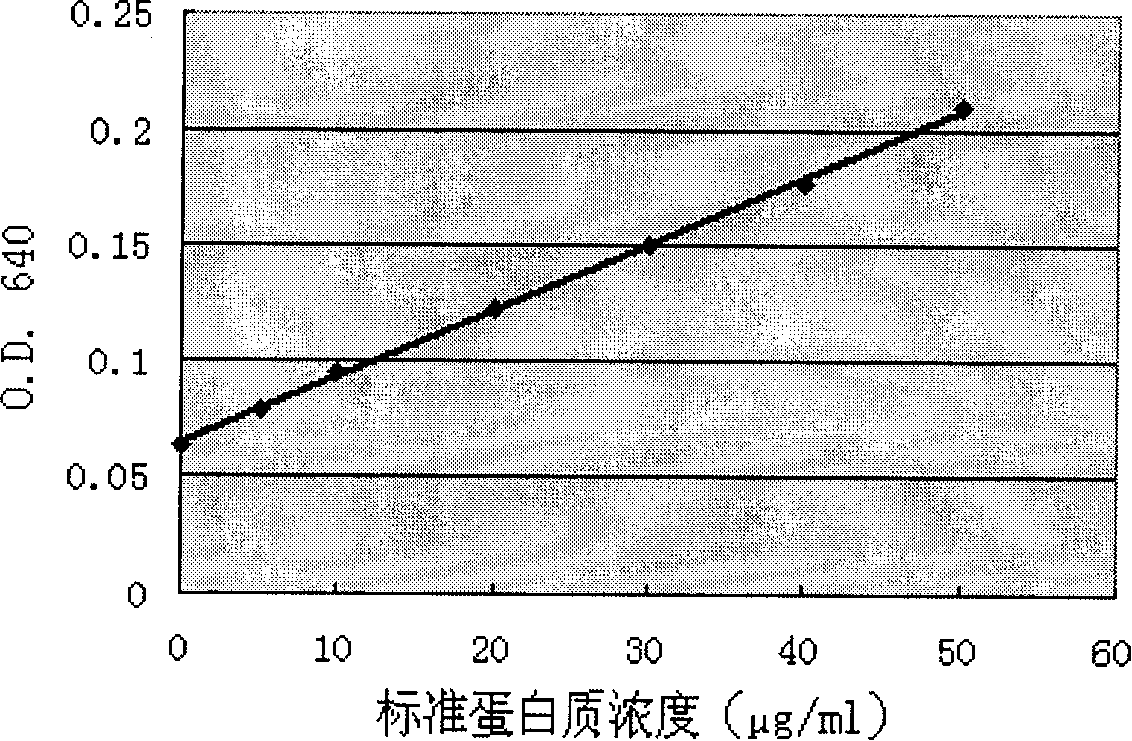
[0061] Example 1: Purification of human sDR5 protein
[0062] Conventional method to activate pGAPZ-containing α A-sDR5 and empty vector pGAPZ α A Pichia strain, and a large number of sDR5-containing Pichia strain GS115 strains (see: Song K, Chen Y, Goke R, et al. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is an inhibitor of autoimmune infection and cell cycle progression. J Exp Med, 2000; 191(7): 1095-1104), take pGAPZ respectively α A-sDR5 and empty vector pGAPZ α A Transformation-positive yeast, cultured for 24 hours, took 3L of supernatant, centrifuged at low temperature and high speed in a Beckman centrifuge (4°C, 8000rpm, 15min), and passed through a hollow fiber ultrafilter (ultrafiltration membrane cut-off flow: 3kDa) Concentrate 20 times by ultrafiltration.
[0063] ProBond TM The affinity chromatography column was passed through 20mmol / L Na 3 PO 4 After equilibrating with 500mmol / L NaCl (pH 7.2-7.6) binding buffer, pass the above sample mi...
Embodiment 2
[0065] Example 2: Preparation of a mouse model of hepatitis B virus, using methods of cell biology and molecular biology, the following experiments were carried out: to study the effect of human sDR5 protein on the liver injury of mice with hepatitis B virus.
[0066] (1) Set up normal BALB / c mouse control group (normal saline control group), seven doses of human albumin control group, seven doses of human sDR5 protein control group, empty vector control group, hepatitis B virus The model group, the treatment group with Minon 24 hours before the establishment of the model, the treatment group with seven doses of human sDR5 protein 24 hours before the establishment of the model, the treatment group with seven doses of human sDR5 protein at the same time after the establishment of the model, refer to sDR5 in mice Dosage used in experimental arthritis [See: Song K, Chen Y, Goke R, et al.Tumor necrosisfactor-related apoptosis-inducing ligand(TRAIL) is an inhibitor of autoimmuneinfl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com