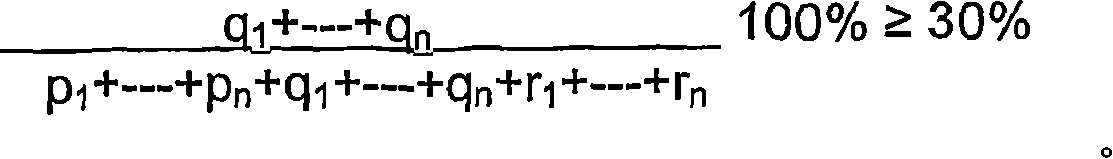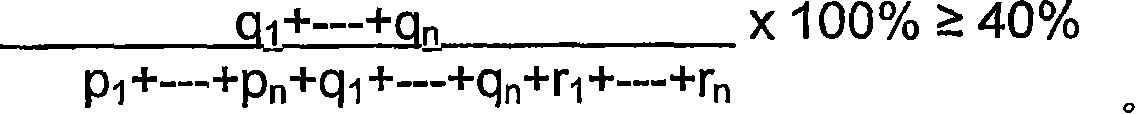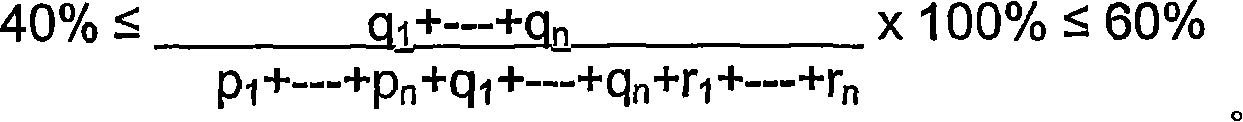Composition for veterinary and medical use
A composition and compound technology, applied in application, drug combination, active ingredient of heterocyclic compounds, etc., can solve problems such as weak antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Example 1 Preparation of compound of general formula (1), berberine chloride
[0079] The roots and bark (1.5kg, ground) of Berberines vulgaris were refluxed with ethanol (10L×2). The ethanol extract was filtered and evaporated under reduced pressure to obtain a brown oil. The residue was dissolved in a warm 0.1M HCl solution (10L×2), filtered, and the filtrate was evaporated to about 1L in vacuo, and then stirred at room temperature overnight. The yellow precipitate was collected and washed with cold water, then re-dissolved in boiling water (10L×2), cooled to room temperature, filtered, washed with water and air dried to obtain yellow crystalline powder of berberine chloride (44.5g). The crystals were analyzed by NMR and the following results were obtained:
[0080] 1 H-nmr(CD 3 OD)×(ppm)
[0081] 3.16(t, 2H), 4.02(s, 3H), 4.15(s, 3H), 4.88(t, 2H), 6.12(s, 2H), 7.03(s, 1H), 7.74(s, 1H), 7.95 (d, AB, 1H), 8.15 (d, AB, 1H), 8.89 (s, 1H), 9.83 (s, 1H).
Embodiment 2
[0082] Example 2 Preparation of compound of general formula (2), 40% oxidized cellulose
[0083] Under the condition of less than 30℃, add a part of microcrystalline cellulose (particle size diameter is about 20) to a stirred aqueous solution (1.08L) of periodic acid (140.7g, 0.6175mol) with pH<0.5 over 2 hours ( 250g, 1.54 moles). The entire mixture was stirred at 30-32°C for 4 hours and then at room temperature for 16 hours. A 5% sodium hydroxide solution (approximately 480 ml) was added to the resulting reaction mixture to adjust the pH to 5-6. The suspension was filtered; the solid (2L×4) was washed with water until the filtrate did not show the presence of an oxidizing agent on the KI-starch test paper. Then the solid was washed with acetone (0.5 L) and air dried to obtain 40% oxidized cellulose (215 g, 87%) as a white powder.
Embodiment 3
[0084] Example 3 Preparation of the compound of general formula (2), 40% oxidized water-soluble cellulose
[0085] Over two hours, sodium periodate (532.5 mg, 2.48 mmol) was added dropwise to a stirred aqueous solution (25 ml) of carboxymethyl cellulose (molecular weight 250,000, DS = 0.7) (1 g, 6.17 mmol) in an ice bath ) Of aqueous solution. The reaction mixture was stirred at 5-10°C for 16 hours, and then dialyzed against water in a dialysis tube (permeation molecular weight limit of about 10,000) for 48 hours. The solution was then freeze-dried to obtain 40% oxidized water-soluble cellulose (813 mg, 81.4%) as a white powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com