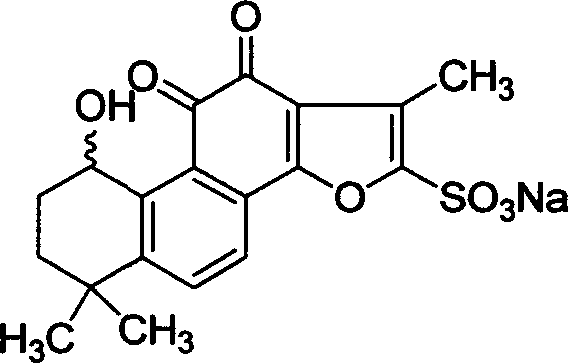
Hydroxy tanshinone IIA sodium sulfonate and its application
A technology of hydroxy Salvia miltiorrhiza and sodium sulfonate, applied in the field of medicine, can solve problems such as effective monitoring
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Example 1: Separation and purification of sodium hydroxytanshinone IIA sulfonate
[0016] Take 10 tanshinone IIA sodium sulfonate water needles with batch number 030340 to evaporate the water, add methanol to dissolve and observe the condition of the plate layer. The chloroform / methanol (7:1) is repeatedly expanded, and it can be seen that the Rf value is slightly lower than that of tanshinone IIA sodium sulfonate by a trace amount. impurity point. Preparative plate separation yielded about 10 mg of orange-red solid, and the compound was further purified by HPLC semi-preparative column to purify the orange-red solid to yield a substance (A).
Embodiment 2
[0017] Example 2: Synthesis of Sodium Hydroxytanshinone IIA Sulfonate
[0018] Dissolve 396 mg (0.001 mol) of sodium tanshinone IIA sulfonate in 10 ml of water, take an ice-water bath, add an appropriate amount of acetic acid dropwise, then add 118 mg (0.001 mol) of chromic acid in batches, stir, and keep the temperature at 5-10 °C for 30 minutes After stopping stirring, the reaction solution was immediately added to 1 liter of saturated sodium chloride aqueous solution, that is, a red solid was precipitated, centrifuged, and the precipitate was washed twice with saturated sodium chloride solution, and then once with an appropriate amount of water, so that the solid ph = 5-6, the filtered solid was subjected to silica gel column chromatography (eluent: CHCl) 3 : CH 3 OH=8:1), collect the desired eluent, evaporate under reduced pressure, and dry to obtain about 200 mg of red crystals.
[0019]
Embodiment 3
[0020] Example 3: Structural confirmation of sodium hydroxytanshinone IIA sulfonate
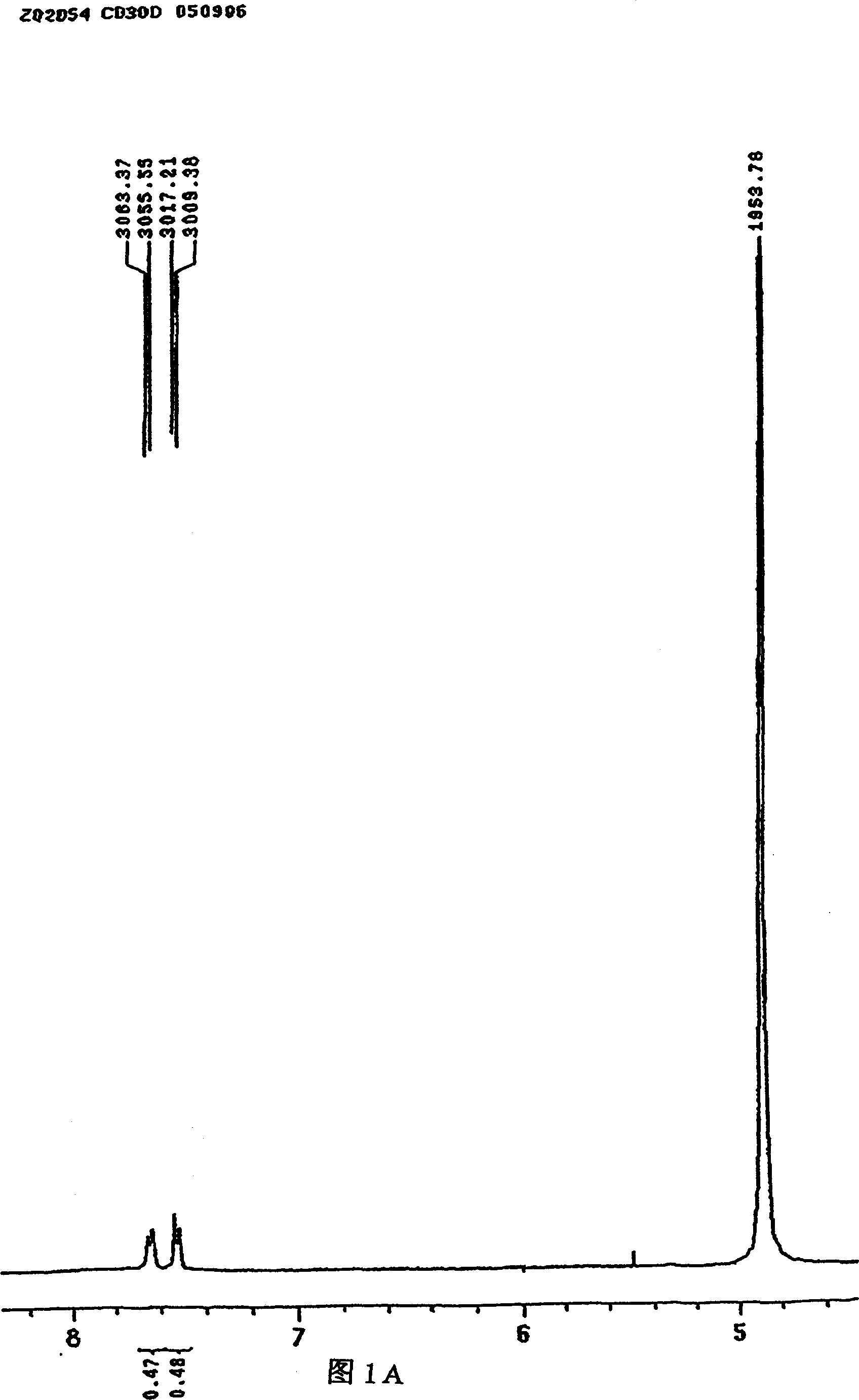
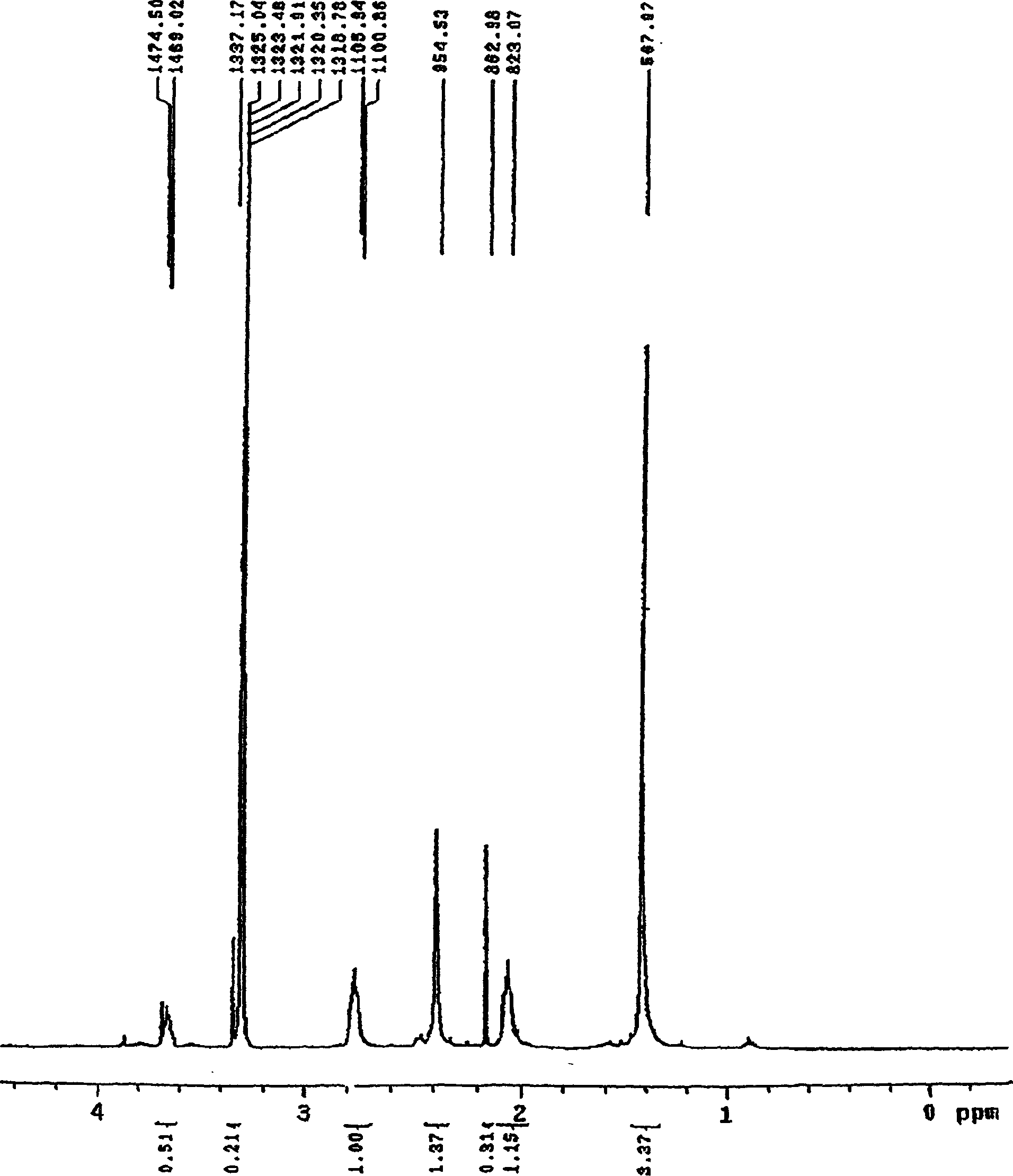
[0021] It is confirmed that the substance (A) obtained in Example 1 has the same structure as the substance synthesized in Example 2, and its hydrogen spectrum is shown in Figure 1A, Figure 1B .
[0022] (Ms: 390(M+1)).
[0023] NMR (CDCl 3 , δ): 1.36 (s, 6H, 2×CH 3 ), 2.1~2.4(m, 4H, CH 2 , CH 2 ), 2.7(s, 3H, CH 3 ), 3.7 (s, H, CH-OH), 7.50 (s, H, CH), 7.7 (s, H, CH).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com