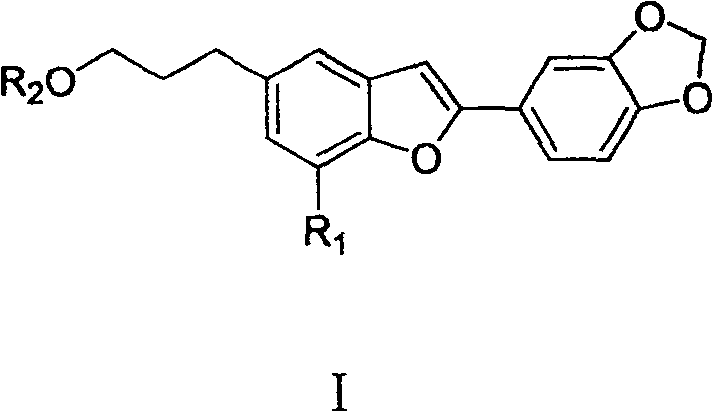
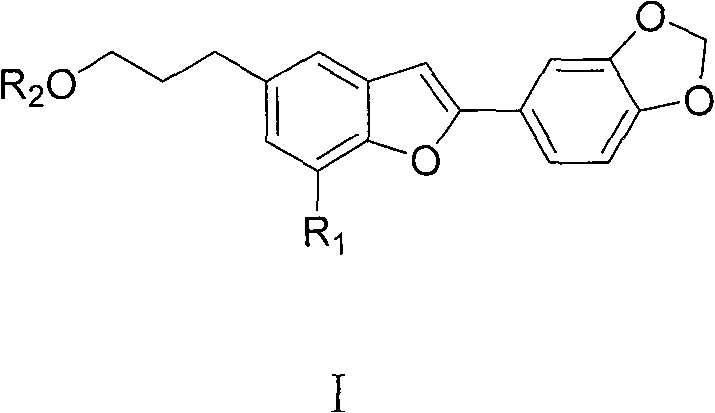
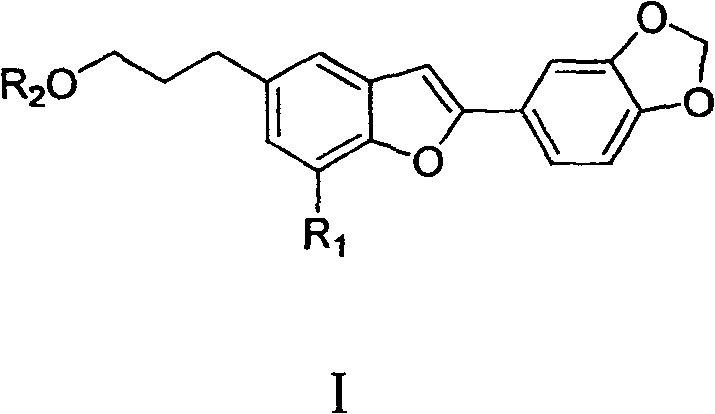
Oleanol type benzofuran and its glucoside applied in preparation of anti female hormone deficiency medicine
A technology of estrogen deficiency and oleanol, which is applied in the field of compounds to achieve remarkable curative effect, promote the synthesis of estrogen, and be convenient to use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Extraction, separation and structure confirmation of oleanol-type benzofuran and its glycosides A, B, C, D, E, F, G, H, I
[0031] Extraction and separation: The seeds of Wasan benzoin were collected from Yongde, Yunnan, and were identified as Styrax perkinsiae. Get 2.2kg (dry weight) of the seeds of Washan Benzoin, pulverize and extract 3 times at room temperature with 80-90% technical alcohol 25L, each for 7 days. The extract was concentrated in vacuo to obtain 550g of the total extract, which was suspended in 5L of hot water, extracted 5 times with petroleum ether, chloroform and n-butanol in sequence, each time with 5L of solvent, and the extract was concentrated respectively to obtain the extract: Petroleum The ether fraction was 16 g, the chloroform fraction was 200 g, and the n-butanol fraction was 120 g. Samples were taken for in vitro activity screening, and both the chloroform and n-butanol fractions showed estrogen-promoting activity. The chlorof...
Embodiment 2
[0053] Example 2: Oleanol-type benzofuran and its glycoside A-I promote the synthesis of estrogen in mouse ovarian granulosa cells:
[0054] Collect mouse ovarian granulosa cells, culture them in a 96-well culture plate, add testosterone or androstenedione (0.5 mM), the raw material required for estrogen synthesis, and add the drug to be tested at a concentration of 100 μg / ml at the same time, at 37 ° C, 5 %CO 2 Cultivate under conditions for 48 hours, collect the supernatant, centrifuge at 1000 rpm for 3min, remove residual cells, and use an enzyme immunoassay kit to detect estradiol (E 2 ) or estrone content, (converted to E with 0.5mM testosterone 2 The maximum value of is taken as 100% formation rate, and the E of the compound is calculated 2 Synthesis rate), the results are shown in the following table 2:
[0055] Table 2. Oleanol-type benzofuran and its glycosides A-I promote the synthesis of E in mouse ovarian granulosa cells 2
[0056]
Embodiment 3
[0057] Example 3: Oleanol-type benzofuran and its glycoside A-I promote the synthesis of estrogen in mouse adipocytes:
[0058] The 3T3-L1 adipocytes of insulin-resistant mice were divided into 2.4×10 5 / well, 1mL / well was inoculated into 24-well culture medium, and E was added after the cells grew into a monolayer 2 Synthesize the desired substrate testosterone (0.5mM), and add the drug to be tested at a concentration of 100 μg / ml at the same time, continue at 37 ° C, 5% CO 2 Conditions were cultivated, and no drug (only testosterone) was used as a control. After 72 hours, the supernatant was drawn, centrifuged at 1000 rpm for 3 min, and residual cells were removed, and the content of estrogen in the supernatant was detected by an enzyme immunoassay kit (converted to E with 0.5mM testosterone). 2 The maximum value of is taken as 100% formation rate, and the E of the compound is calculated 2 Synthetic rate), the results are shown in the following table 3:
[0059] Table 3....
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com