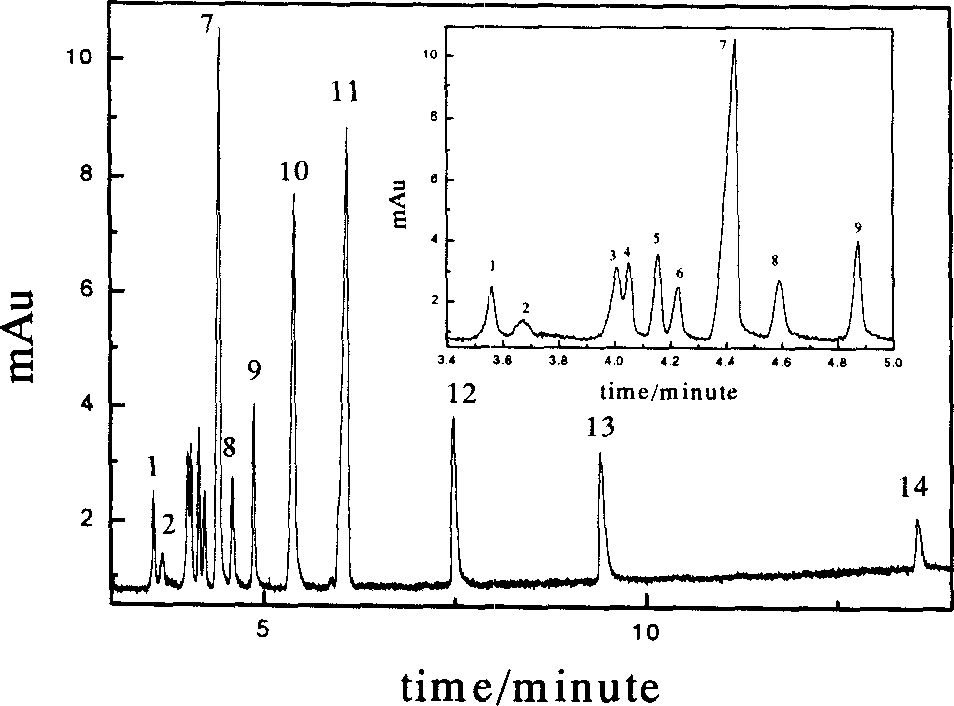
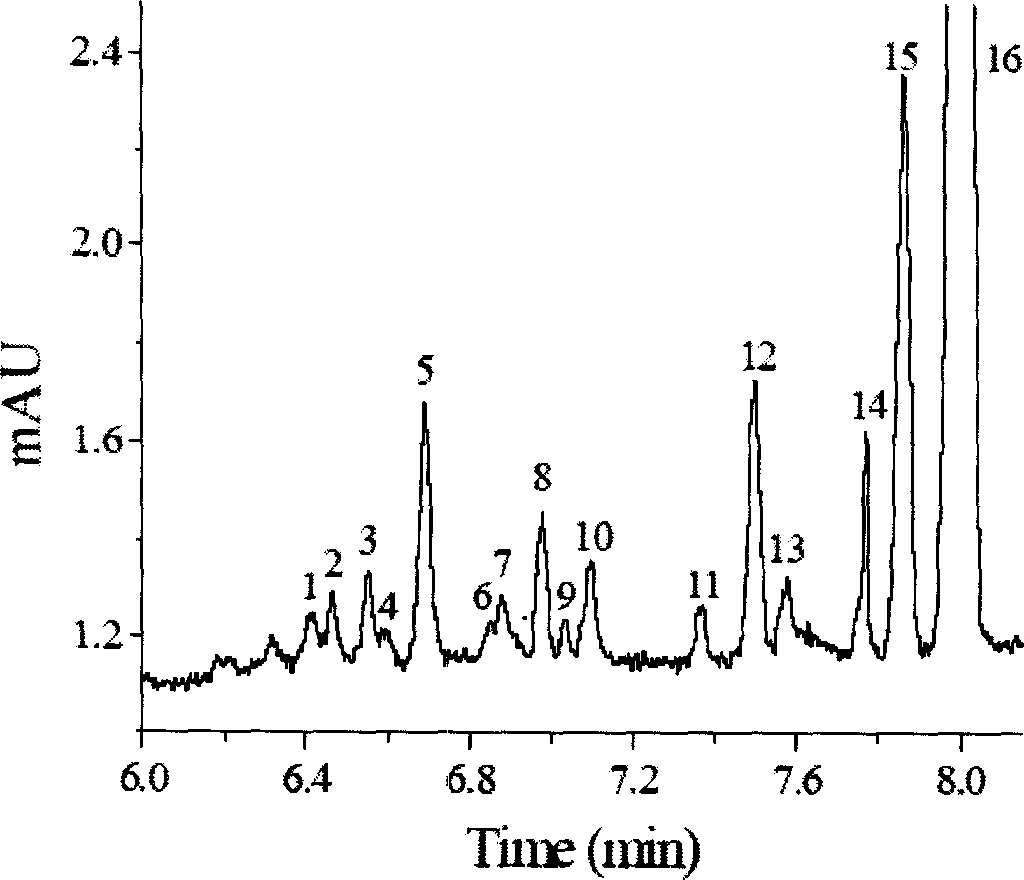
PAABSF column front derivatizing process of amino-acid
A pre-column derivatization, amino acid technology, applied in the field of analytical chemistry, can solve the problems of interference separation, poor reagent selectivity, unstable derivative products, etc., and achieve the effect of simple derivatization reaction conditions and less interference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0010] Instruments and conditions: capillary electrophoresis apparatus, the total length of the capillary is 60cm, the effective column length is 50cm, and the inner diameter is 50μm. 13 kinds of amino acids (biochemical reagents, Sinopharm Group). SDS (biochemical reagent, Alfa Aesar).
[0011] Implementation steps: Quantitatively pipette 40 μL of each amino acid (0.05 mmol / L) into a 2 mL stoppered test tube, add 200 μL of PAABS-F ethanol solution (0.05 mmol / L) and 1 μL of methanol, mix well, and stopper tightly Put it in a constant temperature water bath at 35°C for 30min. Filter with a 0.22 μm membrane before injection.
Embodiment 2
[0013] Instruments and conditions: capillary electrophoresis apparatus, the total length of the capillary is 60cm, the effective column length is 50cm, and the inner diameter is 50μm. 13 kinds of amino acids (biochemical reagents, Sinopharm Group). SDS (biochemical reagent, Alfa Aesar).
[0014] Experimental procedure: Take 0.8mL of the pretreated serum sample, add 200μL of PAABS-F ethanol solution (0.05mmol / L) and 1μL of methanol, mix well, plug it tightly and put it in a constant temperature water bath at 35°C for 30min. Filter with a 0.22 μm membrane before injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com