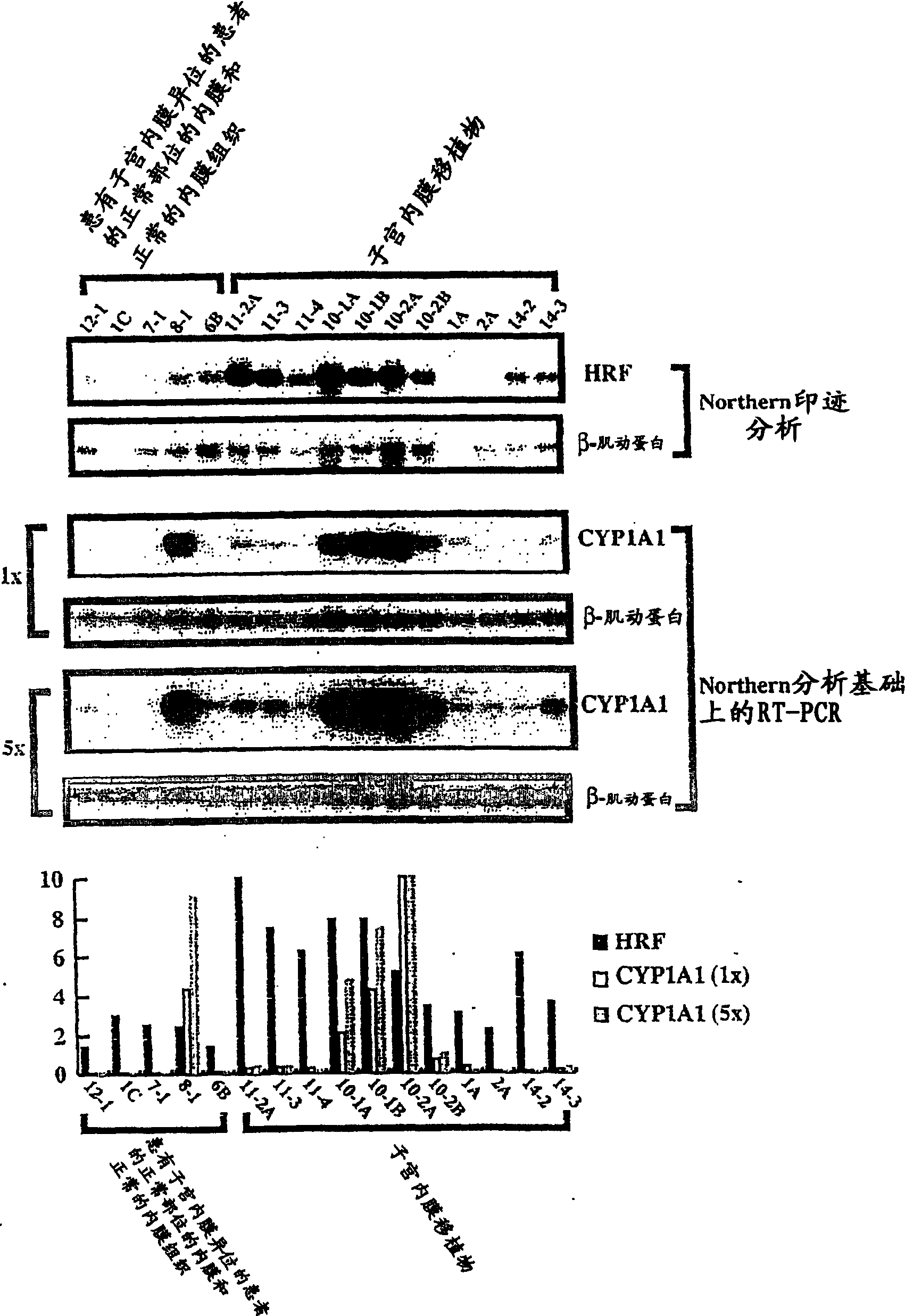
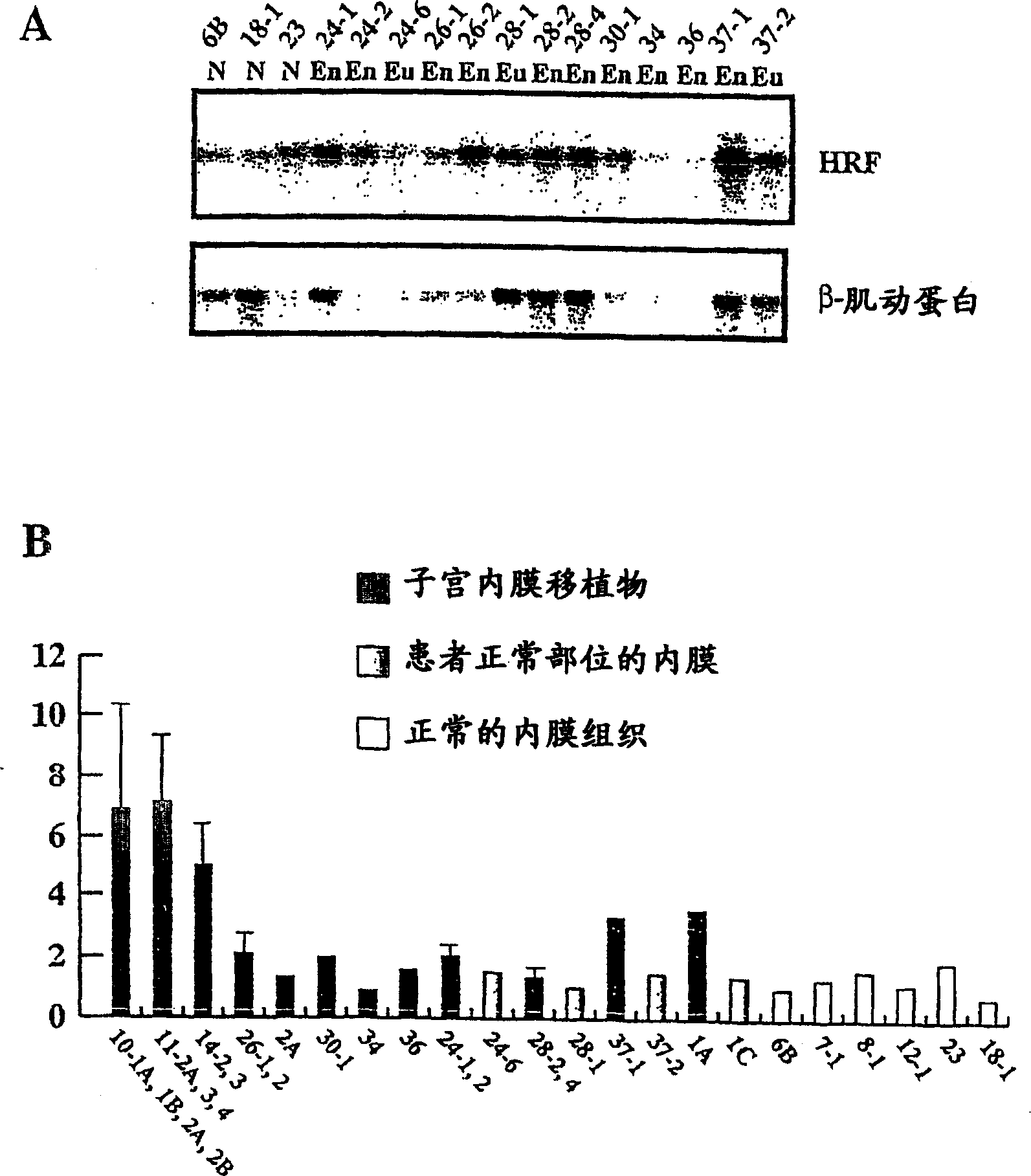
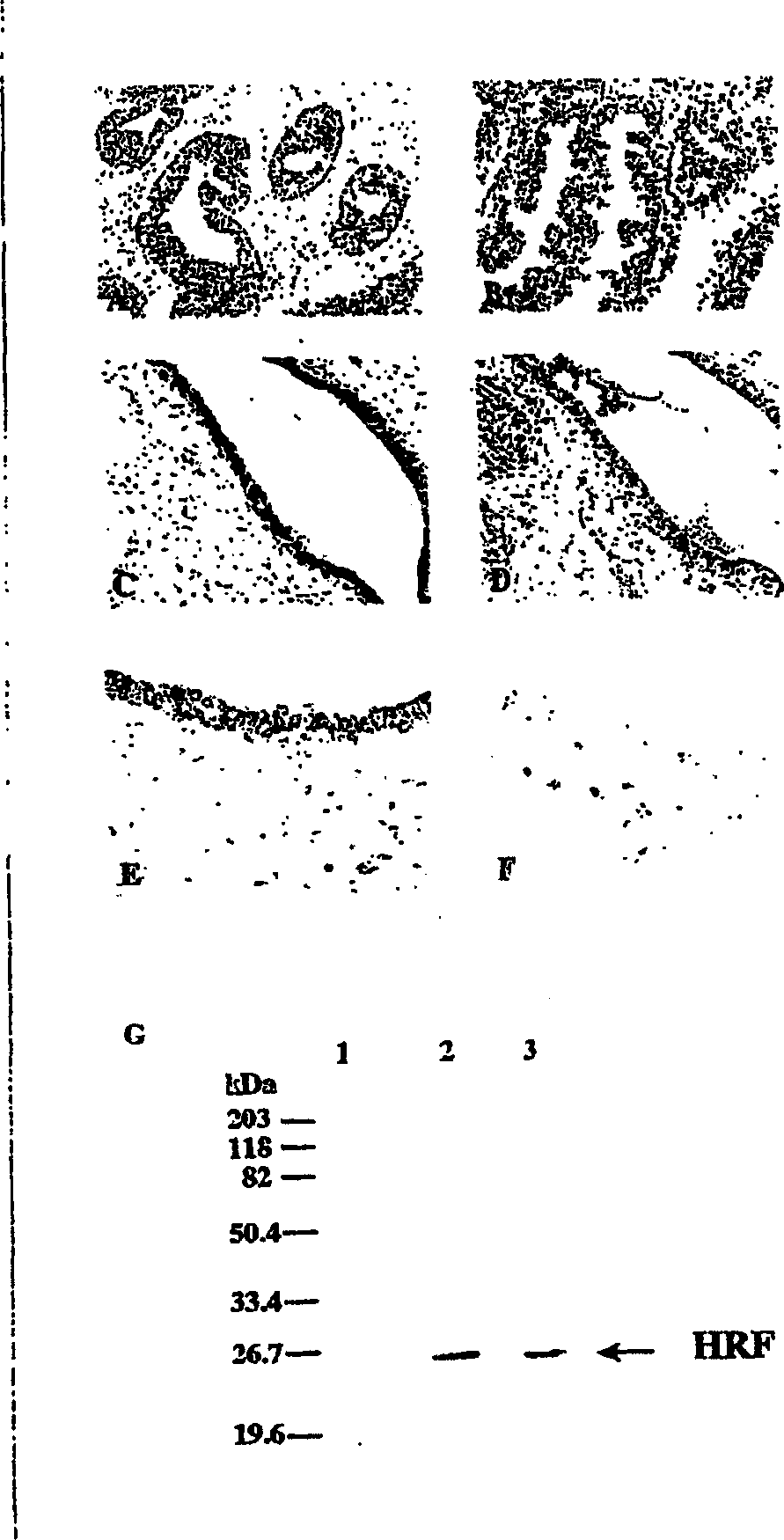
Method for diagnosing endometriosis-related disease in womb
A technology for endometriosis and diagnostic method, which is applied in the field of molecular biology diagnosis and can solve problems such as unclear correlation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0167] Preparation of polyclonal antibodies
[0168] Antiserum containing anti-HRF antibody was prepared, and anti-HRF antibody was purified from the serum.
[0169] As the sensitizing antigen, the peptide at positions 101-116 in the synthetic human HRF protein (SEQ ID NO: 2) can be selected. The synthesized peptide was combined with HLA as a carrier, and then mixed with KLH (50 µg of KLH was added to 50 µg of peptide). The thus obtained antigen solution was mixed with Freund's complete adjuvant to prepare a solution containing the sensitizing antigen. This solution was subcutaneously injected (5 times) into rabbits (SPF Japanese White Rabbit) with a body weight of 3-4 kg in an amount of 1 ml every 2 weeks.
[0170] Then, after the fifth subcutaneous injection, blood was collected from the rabbit at intervals of one week to prepare serum. It was confirmed that the antibody contained in the obtained serum could specifically recognize the HRF protein, and this was used as an ...
Embodiment 3
[0176] Sandwich EIA
[0177] According to the following method, at least one of the anti-HRF antibodies prepared in Example 2 and known anti-HRF antibodies is selected, and the HRF protein is specifically detected and measured by an appropriate combination of the two anti-HRF antibodies, thereby constituting a sandwich EIA system. The EIA system can be a one-step method or a two-step method, and the labeled antibody is not limited to Fab'-HRP. Depending on the purpose of the measurement, the composition of each reaction buffer or the reaction conditions may be adjusted, such as shortening or lengthening. In addition, human HRF used as a standard can be purified from tissue culture supernatant, cell culture supernatant, or recombinants expressed by the content described in Example 1 or other methods. Purification can be achieved by ion exchange, gel filtration, affinity chromatography using an anti-human HRF antibody, or a combination of various other affinity chromatography....
Embodiment 4
[0189] Western blot
[0190] The culture supernatant of cells or tissues expressing human HRF and the purified recombinant human HRF are separated by 10%-15% SDS-PAGE under reducing conditions and transferred to polyvinylidene difluoride (PVDF) membrane (MILLIPORE). Then use TBS (blocking buffer) containing 5% BSA or 5% skimmed milk powder and 0.05% sodium azide to block at room temperature for 0.5-1 hour, treat with HRF-GKL, and incubate at 25°C for 6 hours or less. Each membrane was washed 4 times with TBS (containing 0.05% sodium azide) containing 0.1% Tween 20, and the bound antibody was mixed with HRP-bound anti-rabbit immunoglobulin antibody diluted 1:1000 with blocking buffer at 25°C. React for 1 hour. After the reaction, the membrane was washed 4 times with TBS containing 0.1% Tween20 (containing 0.05% sodium azide), and the bound antibody was detected by Enhanced chemiluminescence (ECL, Amersham Pharmacia).
[0191] In addition, using HRF-TPY, according to the meth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com