Inhibitors of phosphodiesterases in infertility
A technology of inhibitors and preparations, applied in the field of reproductive biology and assisted reproductive technology, can solve the problems of low success rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
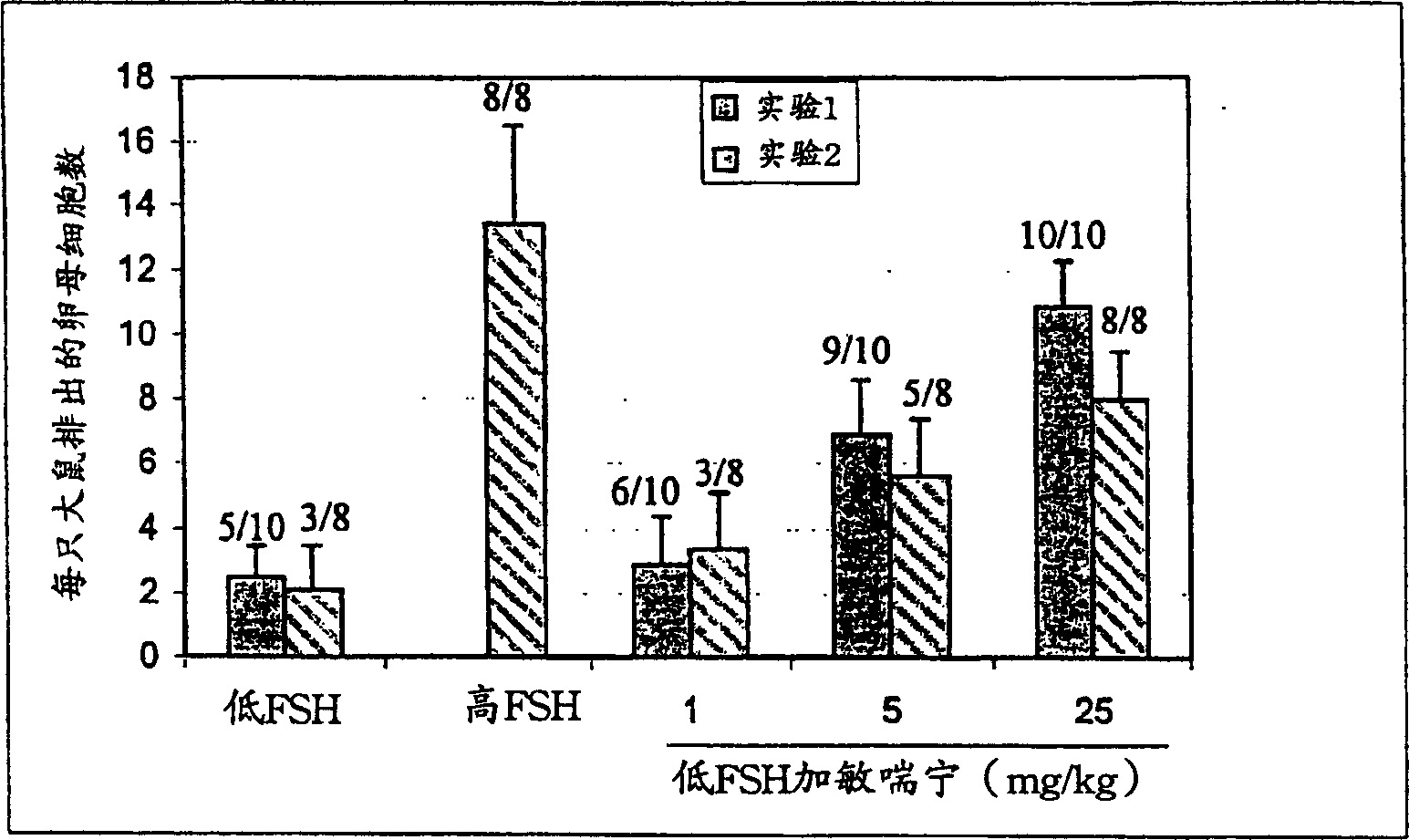
[0157] Example 1: Ovulation induction with various PDE inhibitors combined with low-dose FSH
[0158] The experiment was carried out according to the above-mentioned in vivo rat follicle maturation test. In one experiment, ovaries were collected for histological analysis in the middle of the second day of treatment before the last injection. These rats received only 3 doses of FSH and test compound prior to sacrifice and organ harvesting. Count the total number of secondary follicles, including small follicles (mid-mature follicles with multilayered granulosa and first-order scattered vacuoles but no antral cavity) and antral follicles (those with antral enlargement and an outer diameter of approximately 500 microns or greater, With or without thickened granulocyte layer), evaluate secondary follicles. Antral follicles (≥500mcm) were also counted separately.
[0159] data analysis
[0160] The proportion of ovulating animals, the average number of ova present in the ampull...
Embodiment 2
[0193] Example 2: Various PDE inhibitors in combination with low-dose FSH induce cAMP
[0194] In addition to evaluating the resulting granulosa cells as described above, JC-410 porcine granulosa cells were used for the determination of cAMP in the cell lines. These cells were obtained from Dr. Jorge Chedrese (Sakatchewan University). Cells were maintained in DNEN / F12 supplemented with 5% newborn calf serum (Gibco) and 5 μg / ml insulin (Gibco). Stable cell lines were established by transfection of cDNAs for human LH and FSH receptors into cells using standard transfection techniques and selection with 300 [mu]g / ml Geneticin (Gibco). Cells were maintained in the same concentration of geneticin after selection. For cAMP assays, cells were seeded in 96-well plates at a density of 25,000 cells / well and assayed one day later. The next day, cells were stimulated for 1 hour with increasing amounts of inhibitor molecules in the presence or absence of 1 nM FSH as indicated. All comp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com