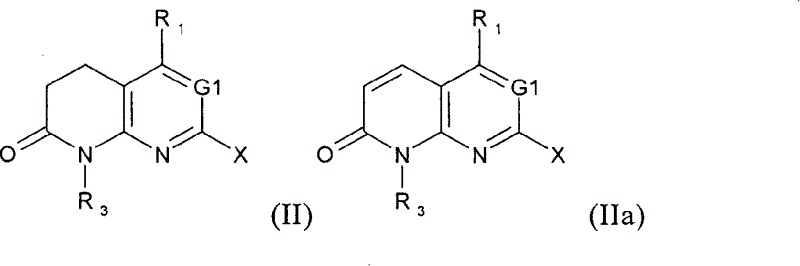
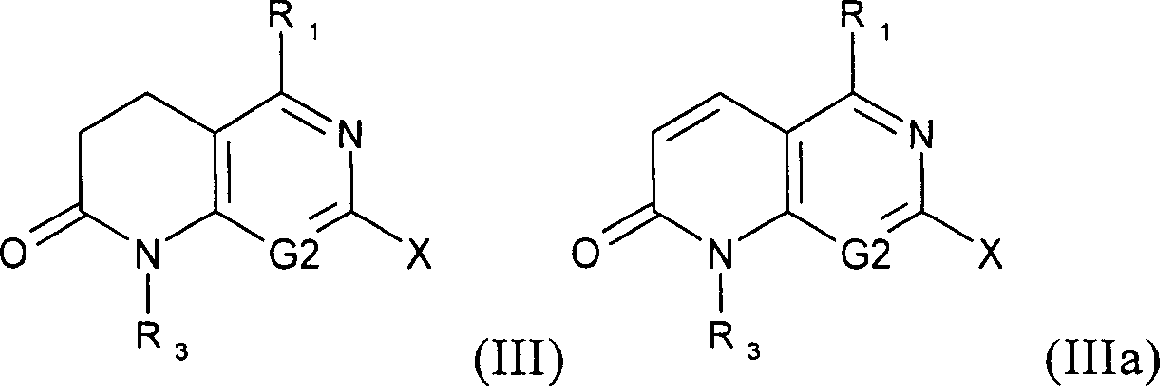
Novel compounds
A compound, alkyl technology, applied in 1 field, can solve the problem of not understanding the signal transduction pathway and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
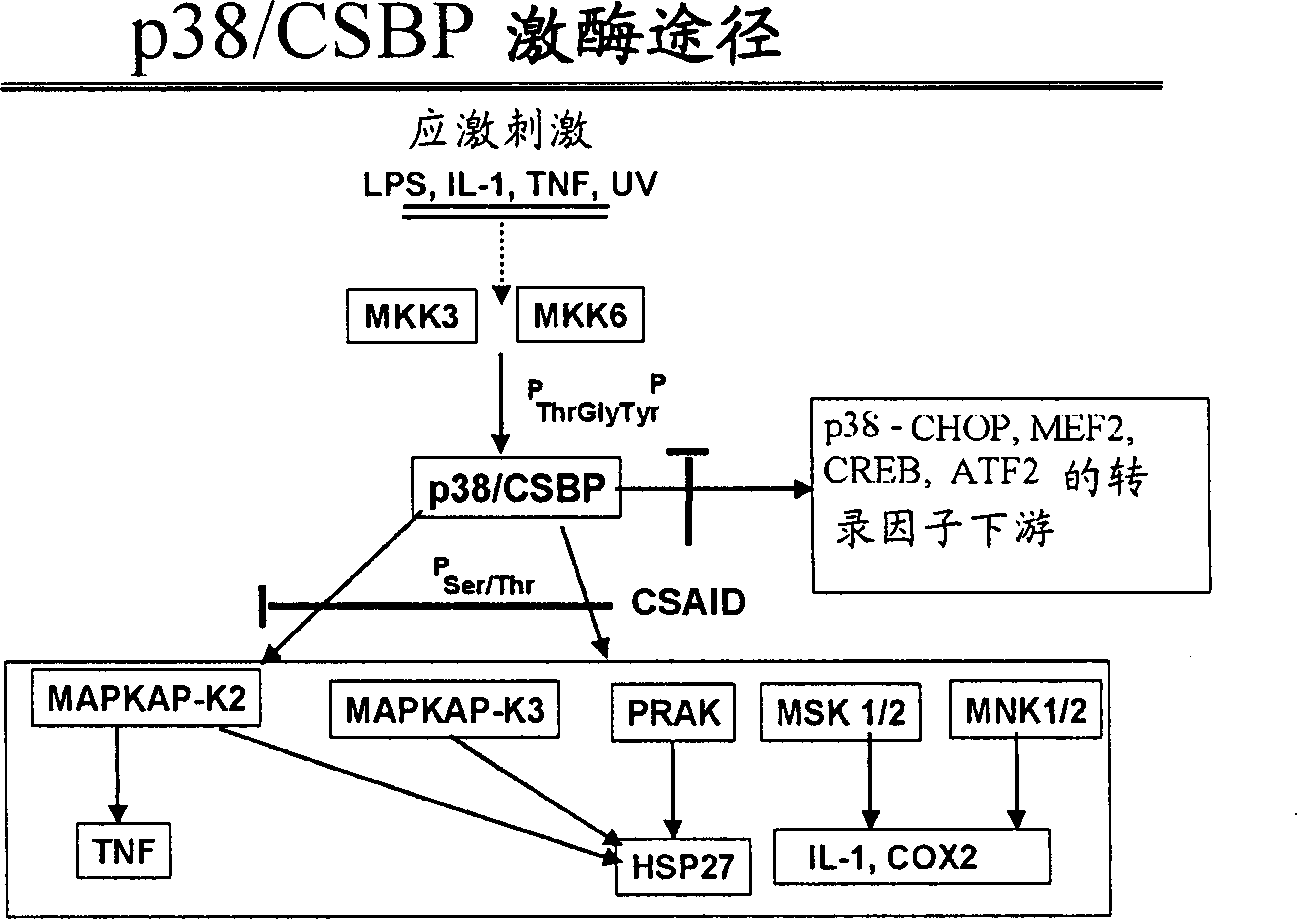
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0257] A series of 2(1H)-quinolones were prepared as described in Scheme 6. The starting material 1-scheme can be prepared from commercially available 1,3-dibromo-5-methoxy-2-methylbenzene by known literature methods such as those disclosed in International Publication No. WO 02 / 058695 A1 6.
[0258] Intermediate 4-Scheme 6 was prepared by two different methods. In the first approach, coupling bromide 1-Scheme 6 with a malonate or monomalonate in the presence of sodium hydride in THF prepared after the desired saponification and decarboxylation Desired compound 2-Scheme 6. Other suitable bases include, but are not limited to, lithium hydride, potassium hydride, sodium ethoxide, butyllithium, which can also be used in suitable organic solvents including, but not limited to, DMF, diethyl ether, dioxane, and ethanol. Carboxylic acid 2-Scheme 6 can then be converted to the corresponding activated carboxylate. Acid chlorides can be prepared, for example, using oxalyl chloride o...
Embodiment 1
[0421] 7-Bromo-1,5-bis(2-chlorophenyl)-3,4-dihydro[1,6]phthalazin-2(1H)-one
[0422]
[0423] 1a) 4,6-dibromo-3-(bromomethyl)-2-(2-chlorophenyl)pyridine
[0424]
[0425] The preparation of the title compound was synthesized as described in WO 02 / 058695, the disclosure of which is incorporated herein by reference in its entirety.
[0426] 1b) tert-butyl 3-[4,6-dibromo-2-(2-chlorophenyl)-3-pyridyl]propionate
[0427]
[0428]Under nitrogen at -10°, a hexane solution of n-butyllithium (1.6 molarity (hereinafter "M"), 3.75 milliliters (hereinafter "ml"), 6.0 millimoles (hereinafter "mmol")) was added Into a solution of di-isopropylamine (0.84ml, 6.0mmol) in dry THF (15ml). After 5 minutes (hereinafter "min"), the solution was cooled to -70° and tert-butyl acetate (0.5 ml, 3.71 mmol) was added dropwise. After stirring at -70°C for 10 minutes, 4,6-dibromo-3-(bromomethyl)-2-(2-chlorophenyl)pyridine (0.405 grams (hereinafter "g"), 0.92 mmol was added under nitrogen ) i...
Embodiment 2
[0446] 7-Bromo-1,5-bis(2-chlorophenyl)[1,6]phthalazin-2(1H)-one
[0447]
[0448] Tetrachloride of 7-bromo-1,5-bis(2-chlorophenyl)-3,4-dihydro[1,6]phthalazin-2(1H)-one (30mg, 0.067mmol) The carbon (5ml) solution was treated with NBS (15mg) and AIBN (1.1mg) and heated at reflux for 1.5h. DBU (10 μl) was added, the mixture was cooled and dichloromethane (5 ml) was added followed by 8% sodium bicarbonate (10 ml). The mixture was passed through a hydrophobic frit and evaporated in vacuo to give the product as an off-white solid.
[0449] NMR (400MHz, CDCl 3 )δ 7.75-7.69 (1H, m), 7.60-7.33 (8H, m), 6.75 (1H, d), 6.65 (1H, s)
[0450] LC / MS R t 3.50 minutes m / z 445 / 447 / 449 [MH + ]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com