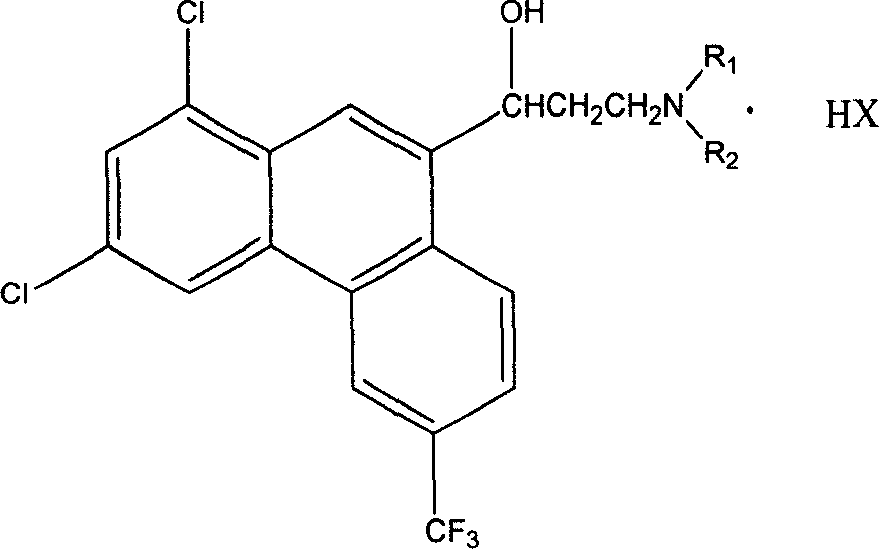
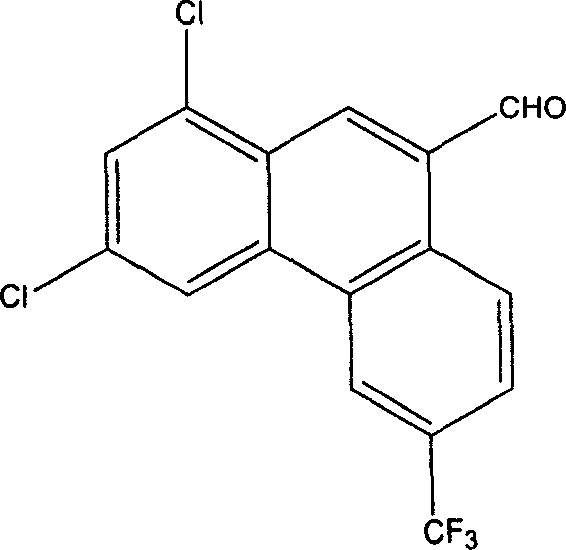
1,3-dichlor-6-trifluomethyl-9-phenanthrene formaldehyde preparation method
A technology of trifluoromethyl and formaldehyde, which is applied in the field of preparation of 1,3-dichloro-6-trifluoromethyl-9-phenanthrene formaldehyde, can solve the problems of harsh conditions, low melting point and low yield, and achieve The effect of mild reaction conditions, simple process operation and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0019] Preparation of active manganese dioxide
[0020] Dissolve 151 grams of manganese sulfate in 2.87 liters of hot water at 60°C, add a solution made of 105 grams of potassium permanganate and 2 liters of water under stirring, stir the mixture at 60°C for 1 hour, filter, and use the filter cake Wash with distilled water until the filtrate is colorless, dry the filter cake at 100-120°C, grind it finely to obtain 120 g, and store it in vacuum. Activate at 200°C for 1 hour before use.
Embodiment 1
[0022] In a 500 ml three-neck flask equipped with mechanical stirring and a thermometer, add 6.9 g of 1,3-dichloro-6-trifluoromethyl-9-phenanthrene methanol (0.02 mol) and 300 ml of dichloromethane, stir until dissolved, Add 13.9 grams (0.16 moles) of active manganese dioxide at 20°C, stir vigorously at 20°C for 24 hours, after the reaction is complete, filter, wash the filter cake with 3×200 ml of dichloromethane, combine the dichloromethane, and evaporate to dryness to obtain light yellow The solid was dried to obtain 6.0 g of the product, with a yield of 87.5%. Melting range 185.4~185.9℃, content 99.5% (HPLC).
Embodiment 2
[0024] Repeat the operation of Example 1, only change the amount of active manganese dioxide in Example 1 to 8.7 grams (0.1 mole), and the reaction time is 40 hours to obtain a product yield of 85.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com